��Ŀ����
��15�֣� ����A��B��C��D��E����ǿ����ʣ�������ˮ�пɵ�������������ӣ��������Ӳ��ظ�����
| ������ | H+��Na+��A13+��Ag+��Ba2+ |
| ������ | OH����C1����CO32����NO3����SO42- |
��֪����A��B����Һ�ʼ��ԣ�C��D��E��Һ�����ԡ���A��Һ��E��Һ��Ӧ�����������г���������A��Һ��C��Һ��Ӧֻ������������������������ͬ������D��Һ������������Һ��Ӧ���ܲ���������Cֻ����D��Ӧ�����������Իش��������⣺
��1��д��DΪ_________________
��2����C��Һ��μ�������������������Ũ�ȵ�A��Һ�У���Ӧ����Һ�и�������Ũ���ɴ�С��˳��Ϊ��_____________________��
��3��д��E��Һ�������B��Һ��Ӧ�����ӷ���ʽ____________________________________��
��4����֪��NaOH��aq��+HNO3��aq��=NaNO3��aq��+H2O��1��;��H=��aKJ��mol-1����д��B��C��ϡ��Һ��Ӧ���Ȼ�ѧ����ʽ___________________________________________________
��5����100 mL0��1 mol��L-1E��Һ�У���μ���35mL 2 mol��L-1NaOH��Һ�����յõ��������ʵ���Ϊ_____��
(1) AgNO3 (2) C(Na+) ��C��Cl-����C��HCO3-�� ��C��OH-����C��H+��
(3)2Al3+ + 3SO42- + 3Ba2+ + 8OH-=== 2AlO2- + 4H2O + 3BaSO4��
(4)Ba��OH��2��aq��+ 2HCl��aq��===Ba��NO3��2��aq��+2H2O��1��;��H=��2aKJ��mol-1
(5)0.01mol
����:��

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�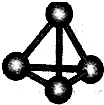 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��






