��Ŀ����
����Ŀ����ѧ����������������������Դ�������Ⱦ���ش��������⣺
(1)NO�ڿ����д������·�Ӧ��2NO(g)+O2(g)![]() 2NO2(g) ��H���÷�Ӧ����������һ����ӦΪ2NO(g)
2NO2(g) ��H���÷�Ӧ����������һ����ӦΪ2NO(g)![]() N2O2(g) ��H1��0����д���ڶ�����Ӧ���Ȼ�ѧ����ʽ����H2�ú���H����H1��ʽ������ʾ����_______________________________________��
N2O2(g) ��H1��0����д���ڶ�����Ӧ���Ȼ�ѧ����ʽ����H2�ú���H����H1��ʽ������ʾ����_______________________________________��
(2)�¶�ΪT1ʱ���������ݻ���Ϊ1 L�ĺ����ܱ������н�������Ӧ��2NO2(g)![]() 2NO(g)+O2(g)������I��5min�ﵽƽ�⡣������������ʾ��
2NO(g)+O2(g)������I��5min�ﵽƽ�⡣������������ʾ��
������� | ���ʵ���ʼŨ��(mol/L) | ���ʵ�ƽ��Ũ��(mol/L) | ||
c(NO2) | c(NO) | c(O2) | c(O2) | |
I | 0.6 | 0 | 0 | 0.2 |
�� | 0.3 | 0.5 | 0.2 | |
���������ڷ�Ӧ����ʼ����_____(������Ӧ�������淴Ӧ��������ƽ�⡱)������С�
�ڴﵽƽ��ʱ������I���������е���ѹǿ֮��Ϊ___________
a. ��1 b. =1 c. ��1

(3)NO2��������ƽ�⣺2NO2(g)![]() N2O4(g) ��H��0����һ��������NO2��N2O4��������������Եķ�ѹ(��ѹ=��ѹ�����ʵ�������)�����¹�ϵ����(NO2)=k1��p2(NO2)����(N2O4)=k2��P(N2O4)����Ӧ�����������ѹ��ϵ��ͼ��ʾ��һ���¶��£�k1��k2��ƽ�ⳣ��kp(ѹ��ƽ�ⳣ������ƽ���ѹ����ƽ��Ũ�ȼ���)���ϵ��k1=______������ͼ������У�ָ���ܱ�ʾ��Ӧ�ﵽƽ��״̬�ĵ���_____��������______________��
N2O4(g) ��H��0����һ��������NO2��N2O4��������������Եķ�ѹ(��ѹ=��ѹ�����ʵ�������)�����¹�ϵ����(NO2)=k1��p2(NO2)����(N2O4)=k2��P(N2O4)����Ӧ�����������ѹ��ϵ��ͼ��ʾ��һ���¶��£�k1��k2��ƽ�ⳣ��kp(ѹ��ƽ�ⳣ������ƽ���ѹ����ƽ��Ũ�ȼ���)���ϵ��k1=______������ͼ������У�ָ���ܱ�ʾ��Ӧ�ﵽƽ��״̬�ĵ���_____��������______________��
(4)����NH3ȥ�� NO���䷴Ӧԭ��4NH3+6NO=5N2+6H2O����ͬ�¶������£�n(NH3)��n(NO)�����ʵ���֮�ȷֱ�Ϊ 4��1��3��1��1��3ʱ���õ�NO�ѳ���������ͼ��ʾ��
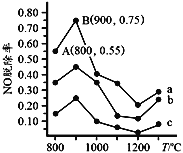
������a�У�NO����ʼŨ��Ϊ6��10-4 mg��m-3����A�㵽B�㾭��0.8 s����ʱ�����NO���ѳ�����Ϊ_______mg/(m3��s)��
������b��ӦNH3��NO�����ʵ���֮����_____��
(5)�����ü�ӵ�ⷨ��NO����ԭ����ͼ��ʾ��

�ٴ�A���г��������ʵ���__________________��
��д�����������ĵ缫��Ӧʽ_______________��
�������ӷ���ʽ��ʾ���ճ��г�ȥNO��ԭ��__________________��
���𰸡�N2O2(g)+O2![]() 2NO2(g) ��H2=��H-��H1 ����Ӧ c 2k2��Kp B��D N2O4��NO2������������������v(NO2)=2v(N2O4) 1.5��10-4 3��1 O2�ͽ�Ũ������ 2HSO3-+2H++2e-=S2O42-+2H2O 2NO+2S2O42-+2H2O=N2+4HSO3-
2NO2(g) ��H2=��H-��H1 ����Ӧ c 2k2��Kp B��D N2O4��NO2������������������v(NO2)=2v(N2O4) 1.5��10-4 3��1 O2�ͽ�Ũ������ 2HSO3-+2H++2e-=S2O42-+2H2O 2NO+2S2O42-+2H2O=N2+4HSO3-
��������
(1)��2NO(g)+O2(g)![]() 2NO2(g) ��H����һ����ӦΪ��2NO(g)
2NO2(g) ��H����һ����ӦΪ��2NO(g)![]() N2O2(g) ��H1��0����ڶ�����ӦΪ��-�ڣ������ɵ�N2O2(g) +O2(g)
N2O2(g) ��H1��0����ڶ�����ӦΪ��-�ڣ������ɵ�N2O2(g) +O2(g)![]() 2NO2(g) ��H2=��H-��H1��
2NO2(g) ��H2=��H-��H1��
(2)�ٶ��ڷ�Ӧ2NO2(g)![]() 2NO(g)+O2(g)����Ӧ�¶�ΪT1����Ӧ��ʼʱc(NO2)=0.6 mol/L��c(NO)=c(O2)=0����Ӧ�ﵽƽ��ʱc(O2)=0.2 mol/L�����������ת����ϵ��֪��ƽ��ʱc(NO2)=0.6 mol/L-2��0.2 mol/L=0.2 mol/L��c(NO)= 2��0.2 mol/L=0.4 mol/L�����¶��»�ѧ��Ӧ��ƽ�ⳣ��K=
2NO(g)+O2(g)����Ӧ�¶�ΪT1����Ӧ��ʼʱc(NO2)=0.6 mol/L��c(NO)=c(O2)=0����Ӧ�ﵽƽ��ʱc(O2)=0.2 mol/L�����������ת����ϵ��֪��ƽ��ʱc(NO2)=0.6 mol/L-2��0.2 mol/L=0.2 mol/L��c(NO)= 2��0.2 mol/L=0.4 mol/L�����¶��»�ѧ��Ӧ��ƽ�ⳣ��K=![]() =0.8�������������н��е�������Ӧ���ڿ�ʼʱ��Ũ����Qc=
=0.8�������������н��е�������Ӧ���ڿ�ʼʱ��Ũ����Qc=![]() ��0.56��0.8����Ӧ������Ӧ������У�
��0.56��0.8����Ӧ������Ӧ������У�
�ڶ��ڷ�Ӧ������NO��O2����2��1��ϵ�����ı䣬���ݸ��������ʵ�Ũ�ȣ�����Ϊ���Ч��ʼ״̬Ϊc(O2)=0��c(NO)=(0.5-0.2��2) mol/L=0.1 mol/L��c(NO2)=(0.3+0.2��2) mol/L=0.7 mol/L����Ӧ��1L�����н��У����������Ũ�Ⱥ�Ϊ��������ʵ����ĺͣ���������I����ʼʱ��������ʵ���n(��)I=0.6 mol��������ʼʱ��������ʵ���n(��)II=0.7 mol+0.1 molspan>=0.8 mol��0.6 mol������ͬ�����������������ʵ�����ѹǿ�����ȣ����Դﵽƽ��ʱ������I������II�е���ѹǿ֮��С��1���ʺ���ѡ����c��
(3)��Ӧ�ﵽƽ��ʱv��=v����v��=k1p2(NO2)��v��=k2p(N2O4)������Ӧ�ﵽƽ�⣬��v(NO2)��=2v(N2O4)��������Kp=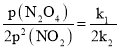 ������k1=2k2Kp������ƽ����v(NO2)=2v(N2O4)����Ϊƽ��㣬����ͼ��B��D����Ӧ�����ʸպ�Ϊ1��2�Ĺ�ϵ������B��DΪƽ��㣻
������k1=2k2Kp������ƽ����v(NO2)=2v(N2O4)����Ϊƽ��㣬����ͼ��B��D����Ӧ�����ʸպ�Ϊ1��2�Ĺ�ϵ������B��DΪƽ��㣻
(4)������a��NO����ʼŨ��Ϊ6��10-4 mgm-3��A����ѳ���Ϊ55%��B����ѳ���Ϊ75%����A�㵽B�㾭��0.8 s����ʱ�����NO���ѳ�����V=![]() =1.5��10-4 mg/(m3s)��
=1.5��10-4 mg/(m3s)��
��NH3��NO�����ʵ����ı�ֵԽ��NO���ѳ���Խ�������ʵ���֮�ȷֱ�Ϊ4��1��3��1��1��3ʱ����Ӧ������Ϊa��b��c��������b��Ӧ�����ʵ���֮����3��1��
(5)�������������ҺΪϡH2SO4������������Һ��ˮ���������OH-ʧȥ���ӷ���������Ӧ���缫��ӦʽΪ��2H2O-2e-=O2��+4H+������ˮ���Ϸŵ����ģ�����������H2SO4��ҺŨ�Ȳ����������Դ�A���г��������ʵ���O2�ͽ�Ũ�����
���ڵ��������ϵõ����ӷ�����ԭ��Ӧ������ͼ��֪��HSO3-�����������·�����ԭ��Ӧ������S2O42-����缫��ӦʽΪ2HSO3-+2e-+2H+=S2O42-+2H2O��
�۸���ͼʾ�����ճ���S2O42-��NO�Ƿ�Ӧ�N2��HSO3-�����������ݵ����غ㡢����غ㡢ԭ���غ㣬�ɵ����ճ��г�ȥNO��ԭ����2NO+2S2O42-+2H2O=N2+4HSO3-��

 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�����Ŀ���ں��������»�ѧ��Ӧ��2SO2(g)��O2(g)![]() 2SO3(g) ��H��-QkJ��mol��1�������������·ֱ���������ͷ�Ӧ�ų�������(Q)������У�
2SO3(g) ��H��-QkJ��mol��1�������������·ֱ���������ͷ�Ӧ�ų�������(Q)������У�
���� | SO2��mol�� | O2��mol�� | N2��mol�� | Q��kJ�� |
�� | 2 | 1 | 0 | Q1 |
�� | 1 | 0.5 | 0 | Q2 |
�� | 1 | 0.5 | 1 | Q3 |
�����������ݣ�����������ȷ���ǣ� ��
A.�����������·�Ӧ����1molSO3�������![]() kJ
kJ
B.2Q1��2Q2��Q1��Q
C.Q1��2Q2��2Q3��Q
D.2Q2��2Q3��Q1��Q