��Ŀ����
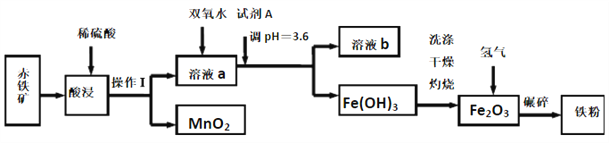
����Ŀ���Գ�������Ҫ�ɷ�Ϊ60.0%Fe2O3,������3.6%FeO��Al2O3��MnO2��CuO�ȣ�Ϊԭ���Ʊ����������ʵ���Ҫ����������ͼ��ʾ��
��֪����������������������ʽ��ȫ����ʱ��Һ��pH�����ʾ, ��ش��������⣺
������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Cu(OH)2 |
pH | 3.4 | 5.2 | 9.7 | 6.7 |
��1���������������õ�MnO2��KClO3��KOH��Һ��Ϲ���,�ɵõ�K2MnO4,�˷�Ӧ�Ļ�ѧ����ʽ��____________________________��
��2����pH������3.6��Ŀ����_______����֪25��ʱ,Ksp[Cu(OH)2]=2��10-20,���¶��·�ӦCu2++2H2O![]() Cu(OH)2+2H+��ƽ�ⳣ��K=_______��
Cu(OH)2+2H+��ƽ�ⳣ��K=_______��
��3������˫��ˮʱ��Ӧ�����ӷ���ʽΪ_______��
��4�����ð�����500�����Ϸֽ�õ��ĵ�ԭ�����������п��Ʊ���������FexNy���������İ���17.0g�����ij�����ʯ1Kg,д���÷�Ӧ�����Ļ�ѧ����ʽ��_______________��
���𰸡� 3MnO2+KClO3+6KOH![]() 3K2MnO4+KCl+3H2O ʹFe3+��ȫ�������������Ӳ����� 5.0��10-9 2Fe2++H2O2+2H+=2Fe3++2H2O 16Fe+2NH3
3K2MnO4+KCl+3H2O ʹFe3+��ȫ�������������Ӳ����� 5.0��10-9 2Fe2++H2O2+2H+=2Fe3++2H2O 16Fe+2NH3![]() 2Fe8N+3H2
2Fe8N+3H2
����������������������Ҫ�ɷ�Ϊ60.0%Fe2O3,������3.6%FeO��Al2O3��MnO2��CuO�ȣ���ϡ�����ܽ⣬����������ת��Ϊ���������ӣ��������̲��ܣ��ټ�˫��ˮ��������������Ϊ�����ӣ�����PH=3.6���������������������ˣ�����������������������������������ԭ�������ʣ�����й����ʵ����ʺ���������⡣
��⣺��1��MnO2��KClO3��KOH��Һ��Ϲ��ȣ��ɵõ�K2MnO4����Ԫ�ػ��ϼ����ߣ�����Ԫ�ػ��ϼ۽��ͣ��������Ȼ��أ����ݵ��ӵ�ʧ�غ��ԭ���غ��֪��Ӧ�Ļ�ѧ����ʽΪ3MnO2+KClO3+6KOH![]() 3K2MnO4+KCl+3H2O��
3K2MnO4+KCl+3H2O��
��2�����ݱ������ݿ�֪��Ϊ��֤������ȫ��������pH����3.4����Ϊ�˲�ʹ�����������ӳ�����pH����̫����pH������3.6��Ŀ����ʹFe3+��ȫ�������������Ӳ���������ӦCu2++2H2O![]() Cu(OH)2+2H+��ƽ�ⳣ��K��c2(H+)/c(Cu2+)��c2(OH-)��c2(H+)/c2(OH-)��c(Cu2+)��K2w/Ksp[Cu(OH)2]��10-28/2��10-20��5��10-9��
Cu(OH)2+2H+��ƽ�ⳣ��K��c2(H+)/c(Cu2+)��c2(OH-)��c2(H+)/c2(OH-)��c(Cu2+)��K2w/Ksp[Cu(OH)2]��10-28/2��10-20��5��10-9��
��3��˫��ˮ�����������ӣ���Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+��2Fe3++2H2O��
��4��1kg������ʯ���������ʵ���Ϊ![]() �����������ʵ���Ϊ17g��17g/mol��1mol�����Ͱ��������ʵ���֮����8��1�����ɵĵ�����ΪFe8N�����������غ㶨�ɿ�֪�÷�Ӧ�Ļ�ѧ����ʽΪ16Fe+2NH3
�����������ʵ���Ϊ17g��17g/mol��1mol�����Ͱ��������ʵ���֮����8��1�����ɵĵ�����ΪFe8N�����������غ㶨�ɿ�֪�÷�Ӧ�Ļ�ѧ����ʽΪ16Fe+2NH3![]() 2Fe8N+3H2��
2Fe8N+3H2��

����Ŀ���о�С������ͼװ����ȡCl2��֤�������������к���HCl��
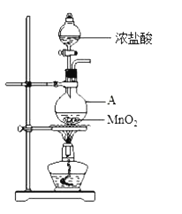
��1������A������Ϊ________��A�з�Ӧ�����ӷ���ʽΪ________��
��2����ͬѧ��A�в���������ͨ��������Һ��
ʵ����� | �Լ� | ���� |
a | ��ɫʯ����Һ | |
b | AgNO3��Һ | ���ְ�ɫ���� |
��ʵ��a�е�����Ϊ________��
������֤�������������к���HCl��ʵ����________������ĸ��ţ���
��3����֪��HCl����ͨ�뱥��ʳ��ˮ���а�ɫ������������ͬѧ��A�в���������ͨ�뱥��ʳ��ˮ�У��а�ɫ��������������ʵ�鲻��֤�������к���HCl����ϻ�ѧ���������ԭ��______________________________________��
��4����֪��2S2O32-+I2=== S4O62-+2I-����ͬѧ��A�в���������ͨ������ˮ�У��õ���ҺX����������ʵ��֤�������к���HCl��
I���ⶨX���ܽ��Cl2��ȡ25.00 mL��ҺX���������KI��Һ��Ȼ����0.04 molL-1 Na2S2O3��Һ�ζ����ɵ�I2����ζ��յ�ʱ����Na2S2O3��ҺV mL��
II���ⶨX��ClԪ����������ȡ25.00 mL��ҺX��ѡ���ʵ��Ļ�ԭ�����ܽ��Cl2ȫ����ԭΪCl-������0.10 molL-1 AgNO3��Һ�ζ�������Һ�е�Cl-��
��X�е�HClO����Ӱ��I�IJⶨ�����ԭ����________��
����I��II ��ʵ�����ݿ�֤��A�в����������к���HCl����II������0.10 molL-1 AgNO3��Һ�����Ӧ����________mL���ú�V�Ĵ���ʽ��ʾ����
����Ŀ��ij��ѧ�С�鰴��ͼ��ʾ�����ɴ�����ͭ��Ʒ(�����������������������������)��ȡ��ˮ����ͭ��

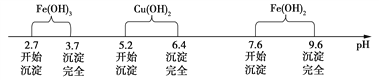
��֪Fe3����Cu2����Fe2������������ˮ��Һ���γ��������������pH��Χ����ͼ��ʾ��
��ش��������⣺
(1)������ʵ������У�����ʵ��װ�ò������õ�����________(�����)��
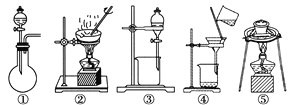
(2)��ҺA����������Ϊ________������XӦѡ��________(�����)��
����ˮ������˫��ˮ���������ۡ����ܸ������
(3)����ҺC����ȡ����ͭ�����ʵ�����Ϊ______________________________��
(4)�á���ӵ����������Բⶨ��ҺA(��������I��������Ӧ������)��Cu2����Ũ�ȡ��������£�
��һ������ȡ10.00 mL��ҺA��100 mL����ƿ�У���ˮ������100 mL��
�ڶ�����ȡϡ�ͺ���Һ20.00 mL����ƿ�У��������KI���壬��ַ�Ӧ���ɰ�ɫ������ⵥ�ʡ�
���������Ե�����ҺΪָʾ������0.050 00 mol��L��1��Na2S2O3����Һ�ζ���ǰ�ⶨ���Σ��ﵽ�ζ��յ�ʱ������Na2S2O3����Һ��������±���(��֪��I2��2S2O![]() ===2I����S4O
===2I����S4O![]() )
)
�ζ����� | ��һ�� | �ڶ��� | ������ |
�ζ�ǰ����(mL) | 0.10 | 0.36 | 1.10 |
������(mL) | 20.12 | 20.34 | 22.12 |
��CuSO4��Һ��KI�ķ�Ӧ�����ӷ���ʽΪ______________________________��
�ڵζ��У�Na2S2O3����ҺӦ����________(���ʽ�ζ��ܡ���ʽ�ζ��ܡ�)�У��ζ��յ��������______________________________��
����ҺA��c(Cu2��)��________mol��L��1��