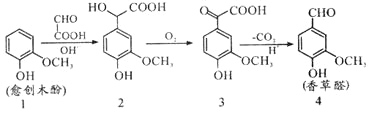
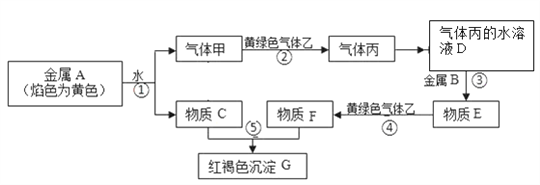
��Ŀ����
����Ŀ��ʳ�ö�������Ư����ʳƷ��������ĸΡ��������������ij����С�����ʵ�������������Ư���ԡ��ش��������⣺
(һ)����������Ʊ�
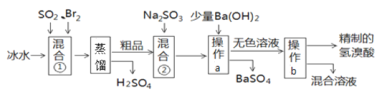
ʵ����һ�������������������(Ũ������ˮ1:1���)��Ӧ��ȡ��������
(1)д��ʵ������SO2�Ļ�ѧ����ʽ________________________________;
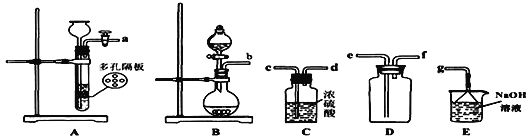
(2)���ռ�һƿ����Ķ�������ѡ����ͼ�е�װ�ã����������˳��Ϊ��_______________________________________(������������Сд��ĸ��ʾ)��
(��)�����������ʵļ���
�������ռ�����SO2ͨ������װ���У���һ���¶��°�ͼʾװ�ý���ʵ�顣
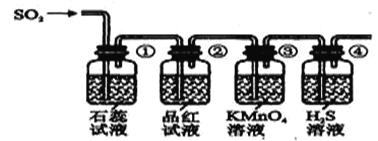
(2)��������ʵ�飬�ش��������⣺
��� | ʵ������ | ����ԭ�� |
�� | _________________________ | _________________________ |
�� | Ʒ����Һ��ɫ | SO2����Ư���� |
�� | ________________________ | ��Ӧ�����ӷ���ʽ____________________ |
�� | ��Һ����ǣ��л�ɫ�������� | SO2+2H2S=3S��+2H2O |
(3)��SO2Ư����ʳƷ��һ��������������Σ�����С������˼���ʳƷ���Ƿ����������εļ�������ʳƷ������ĩ�����ձ�������������ˮ������һ�������ȴ�����£��μ�������Լ�A���۲�Һ�����ɫ�仯�ȿɡ�
�������������Լ�A��_____________��
�ڼ��ȵ������Ǽӿ췴Ӧ����(��ӿ�ʳƷ���ܽ�)������ʱ�䲻��̫����ԭ����_________________________________________________________________��
���𰸡�Na2SO3 + H2SO4=Na2SO4 + H2O + SO2�� b��c��d��e��f��g ʯ����Һ��� ʯ����Һ���˵��SO2���������� KMnO4��Һ��ɫ��ɫ 5SO2+2H2O+2MnO4-=2Mn2++5SO42-+4H+ Ʒ����Һ ��ֹ�������α�����
��������
��һ�������������������ȡSO2���Լ�Ϊ��̬��Һ̬����Ӧ����������ȣ���ͨ������������������������Ʒ�Ӧ���ʣ�ѡ��װ��B�Ʊ�������������Ũ���������ռ�����������Ϊ�к����壬����Ӧע��β��������
��������2�����ݶ�����������ʣ�Ϊ�������壬��ʹʯ���ɫ����Ư����ʹƷ����ɫ�����л�ԭ�ԣ���ʹ���Ը��������ɫ���Ҿ��������ԣ��������Ʒ�����̬���з�Ӧ���ɵ�����
��3�����ݶ�����������ʼ��鼴�ɡ�
��һ����1�������������������ȡSO2���ʷ���ʽΪ��Na2SO3 + H2SO4=Na2SO4 + H2O + SO2����
��2�������������������ȡSO2���Լ�Ϊ��̬��Һ̬����Ӧ����������ȣ���ͨ������������������������Ʒ�Ӧ���ʣ�ѡ��װ��B��������������Ũ���������ռ���Ϊ���ﳹ�ף�����b����Cװ�õ�c�����������ܶȱȿ����������ռ�ʱ������Ӧ�����̳�����������Ϊ�к����壬����Ӧ�������������ն���Ķ����������壬������˳��Ϊ��b��c��d��e��f��g��
�ʴ�Ϊ��b��c��d��e��f��g��
��������2����������Ϊ�������壬������ʹ����ʯ����Һ��죬����Ư���ԣ���ʹ����Ʒ����Һ��ɫ�����л�ԭ�ԣ�������Ӧ5SO2+2H2O+2MnO4-=2Mn2++5SO42-+4H+����ʹ�������Ը��������ɫ�����������ԣ�����������Ʒ�����̬���з�Ӧ�������ʣ�SO2+2H2S=3S��+2H2O��������Һ���ǣ����ɵ���ɫ������
�ʴ�Ϊ��ʯ����Һ��죻ʯ����Һ���˵��SO2���������壻KMnO4��Һ��ɫ��ɫ�� 5SO2+2H2O+2MnO4-=2Mn2++5SO42-+4H+��
��3�������������Ư���ԣ�����Ϊ���γɵ���������Ư���ԣ������������εĴ��ڣ�����Ʒ����Һ�������Ƿ���Ư���Լ��ɣ�����ʳƷ���Ƿ����������εļ�������ʳƷ������ĩ�����ձ�������������ˮ������һ�������ȴ�����£��μ������Ʒ����Һ���۲�Һ�����ɫ�仯�ȿɣ�Ϊ�ӿ췴Ӧ���ʣ����ȣ��������������ױ������е������������ʼ���ʱ�䲻��̫����
�ʴ�Ϊ���ٷ�ֹ�������α���������Ʒ����Һ��

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�����Ŀ������SO2ͨ�벻ͬ��Һ�е�ʵ���������ý��۲���ȷ���ǣ� ��
��Һ | ���� | ���� | |
A | ��HCl��BaCl2��FeCl3��Һ | ������ɫ���� | SO2������������ |
B | H2S��Һ | ������ɫ���� | SO2�������� |
C | ����KMnO4��Һ | ��ɫ��Һ��ɫ | SO2�л�ԭ�� |
D | Na2SiO3��Һ | ������״���� | ���ԣ�H2SO3��H2SiO3 |
A.AB.BC.CD.D