��Ŀ����
����Ŀ����������ҽҩ��ʯ����ҵ���й㷺����;����ͼ�ǹ�ҵ�Ʊ��������Ʒ�����Ƶ����̣����е㣺Br2��58.5����HBr����66.8����H2SO4��338����

��1����Ϣ��з�Ӧ�����ӷ���ʽ��__________________________________��
��2����Ϣ��в�ֱ����ˮ��ʹ�ñ�ˮ��Ŀ����________________________��
��3������b������______�����ķ��루����ţ���
A�������Һ�� B��������� C���������ܵ�Һ�� D�����ܵ�Һ��
��4����Ϣ��м����������Ƶ�Ŀ����__________________��
��5��������������ӦΪ��ɫҺ�壬����ҵ�������Ƶõ������ᣨ��ҵ�����ᣩ�����л�ɫ����ͬѧ�²�����Ǻ���Fe3������ͬѧ�²�����Ǻ���Br2����д��֤ʵ��ͬѧ�����ʵ�鷽����________________________��
��6������ʼʹ��m kg�嵥����ԭ�ϣ���������õ�������������Һ250 L��ȡ2.5 mL������Һϡ�ͣ���ϡ��Һ�м�����������������Һ��ַ�Ӧ������ַ�Ӧ��õ�n g����ɫ�������������������Ϊ__________��
���𰸡�SO2��Br2��2H2O = 4H����2Br����SO42�� ������ϵ�¶ȣ���ֹ��ӷ������ԭ�ϵ������� D ��ȥ��Ʒ�е��� ȡ��Ʒ��������������۲��ϲ��Ƿ��гȻ�ɫ 2000n/47m��100%
��������
��������̱Ƚ�ֱ�ۣ�������![]() �Ļ�ԭ�����Ʊ�
�Ļ�ԭ�����Ʊ�![]() �������� �����ӷ�������ȡ�ӷ����ᡱ��ԭ�������
�������� �����ӷ�������ȡ�ӷ����ᡱ��ԭ�������![]() ���ٽ���һϵ�е��ᴿ���ɣ��Ѷ�һ�㡣
���ٽ���һϵ�е��ᴿ���ɣ��Ѷ�һ�㡣
��1����Ϣټ���������͵�����֮���������ԭ��Ӧ��ע�����岻�ܲ�![]() ��
��
��2������һ�����ӷ���Һ�壬����ñ�ˮ������Ӧ���ʿ�����Ч������Ļӷ������ԭ�������ʣ�
��3������b�����Ƶ�������������Һ���룬�ٽ����ɸ��ķе��֪������b�������������������������ֻ����ҷе����ϴ��Һ�壬���ѡD��
��4���������ƾ��л�ԭ�ԣ������ɸ��ķе��֪������е�ϵͣ�������ʱ������Ʒ�У���˿ɼ�������������ͨ��������ԭ�ķ�����ȥ�����壻
��5����Ҫ֤ʵ��Һ���Ƿ��е����壬��ķ�����ȡ������Ʒ���뱽�������ϲ㱽�����гȻ�ɫ�����֤����Ʒ���е����壻
��6��n�˵���ɫ������![]() mol�廯����2.5mL��Һ����
mol�廯����2.5mL��Һ����![]() mol�廯�������250L�����������о���
mol�廯�������250L�����������о���![]() mol�����ӣ���Щ��Ʒ�е���һ����
mol�����ӣ���Щ��Ʒ�е���һ����![]() kg�����������Ϊ
kg�����������Ϊ![]() �����ɡ�
�����ɡ�

����Ŀ��ʳ�ö�������Ư����ʳƷ��������ĸΡ��������������ij����С�����ʵ�������������Ư���ԡ��ش��������⣺
(һ)����������Ʊ�
ʵ����һ�������������������(Ũ������ˮ1:1���)��Ӧ��ȡ��������
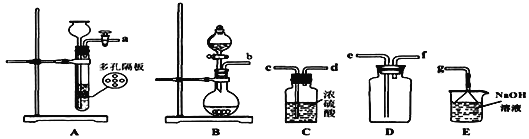
(1)д��ʵ������SO2�Ļ�ѧ����ʽ________________________________;
(2)���ռ�һƿ����Ķ�������ѡ����ͼ�е�װ�ã����������˳��Ϊ��_______________________________________(������������Сд��ĸ��ʾ)��
(��)�����������ʵļ���
�������ռ�����SO2ͨ������װ���У���һ���¶��°�ͼʾװ�ý���ʵ�顣

(2)��������ʵ�飬�ش��������⣺
��� | ʵ������ | ����ԭ�� |
�� | _________________________ | _________________________ |
�� | Ʒ����Һ��ɫ | SO2����Ư���� |
�� | ________________________ | ��Ӧ�����ӷ���ʽ____________________ |
�� | ��Һ����ǣ��л�ɫ�������� | SO2+2H2S=3S��+2H2O |
(3)��SO2Ư����ʳƷ��һ��������������Σ�����С������˼���ʳƷ���Ƿ����������εļ�������ʳƷ������ĩ�����ձ�������������ˮ������һ�������ȴ�����£��μ�������Լ�A���۲�Һ�����ɫ�仯�ȿɡ�
�������������Լ�A��_____________��
�ڼ��ȵ������Ǽӿ췴Ӧ����(��ӿ�ʳƷ���ܽ�)������ʱ�䲻��̫����ԭ����_________________________________________________________________��