��Ŀ����
13����������п��һ�ֶ�ܵ����������ϣ�ijС���Դ�����п��������ͭ�������Ϊԭ��ģ�ҵ������������п��������ͼ��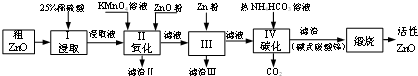
��֪����������������pH��Χ���±���ʾ��
| Zn��OH��2 | Fe��OH��2 | Fe��OH��3 | Cu��OH��2 | |
| ��ʼ����pH | 5.4 | 7.0 | 2.3 | 4.7 |
| ��ȫ����pH | 8.0 | 9.0 | 4.1 | 6.7 |
��1������I������25%ϡ�������98%Ũ���ᣨ�ܶ�Ϊ1.84g/mL�����ƣ���������������������ձ�����Ͳ�⣬����ҪD��ѡ���ţ�
A����ƽ��������B���ζ��ܡ�����C������ƿ��������D����ͷ�ι�
��2���������ͨ������KMnO4��������MnO4-+5Fe2++8H+=5Fe3++Mn2++4H2O�������ӷ���ʽ��ʾ����
��3����ZnO����pH�����Գ�ȥ�������ʣ�����Fe3+�Ƿ������ȫ��ʵ�������ȡ������Һ���μ�KSCN��Һ������ɫ�仯��˵��������ȫ����Ӧ��������ȫ������pH�����˷�Χ��4.1��4.7��
��4��������м���Zn�۵������ǣ��ٳ�ȥ��Һ�е�Cu2+���ڽ�һ��������ҺpH��
��5������IV��ʹ����NH4HCO3��Һ�ܴٽ�Zn2+ת��Ϊ���������¶Ȳ��˹��ߣ���ԭ�������NH4HCO3�ֽ����ʧ��
��6�������·����ⶨ���û�������п�Ĵ��ȣ�
��ȡ1.000g��������п����15.00mL��1.000mol/L������Һ��ȫ�ܽ⣬���뼸�μ��ȣ�
����Ũ��Ϊ0.500mol/L�ı�����������Һ�ζ�ʣ�����ᣬ�����յ�ʱ��������������Һ12.00mL��
�жϵζ��յ�ķ�������Һ��ɫ�ɺ�ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ�����û�������п�Ĵ���Ϊ97.2%��
��7����֪�����£�CuS���ܶȻ�����Ksp=6.0��10-36�����ʵ���Ũ��Ϊ3.0��10-3mol•L-1Na2S��Һ��������CuSO4��Һ�л�Ϻ�����������������CuSO4��Ũ����СΪ4.0��10-33mol•L-1��
���� ������п��������ͭ�����������ϡ�����ܽ⣬���ˣ��õ���Һ�к�����������������ͭ������п��������������Һ������������Ϊ�����ӣ��ټ�������п������ҺpHʹ��Һ��������ת���������˳�ȥ��������Һ����п���Խ�һ��������ҺpH������ԭͭ����Ϊͭ���ʣ����˳�ȥ���õ�����п��Һ������̼�����̼�����ɼ�ʽ̼��п�����ȷֽ����ɻ�������п��
��1����������һ��������������Һ����Һ���ƹ�������Ҫ�������У����������ձ�����Ͳ����ͷ�ιܣ�
��2��������������Һ������������Ϊ�����ӣ���������ԭΪ�����ӣ�ͬʱ����ˮ��
��3�������������������������Һ���ɫ�����֤���������ӳ�����ȫ��ͭ���ӿ�ʼ����pHѡ��
��4������III�м���Zn�۽�һ��������ҺpH������ԭͭ����Ϊͭ���ʣ����˳�ȥ��
��5���¶ȹ��ߣ�NH4HCO3���ֽ⣻
��6���ζ��յ���Һ��ɫ�ɳ�ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ�����������������Ƽ���������ZnO��Ӧ��ʣ�����������ʵ������ټ�����ZnO��Ӧ�����������ʵ���������ZnO+2H+=Zn2++H2O����ZnO�����������������������п�Ĵ��ȣ�
��7�������Ϻ���Һ��������Ũ�ȣ��ٸ���Ksp��CuS��=c��Cu2+����c��S2����������ǻ����Һ��c��Cu2+��������ϡ�Ͷ��ɣ���֪����ͭ��ҺŨ�ȵ��ڻ����Һ��ͭ����Ũ��2����
��� �⣺������п��������ͭ�����������ϡ�����ܽ⣬���ˣ��õ���Һ�к�����������������ͭ������п��������������Һ������������Ϊ�����ӣ��ټ�������п������ҺpHʹ��Һ��������ת���������˳�ȥ��������Һ����п���Խ�һ��������ҺpH������ԭͭ����Ϊͭ���ʣ����˳�ȥ���õ�����п��Һ������̼�����̼�����ɼ�ʽ̼��п�����ȷֽ����ɻ�������п��
��1������I������25%ϡ�������98%Ũ���ᣨ�ܶ�Ϊ1.84g/mL�����ƣ��Ǵ�������һ��������������Һ�����ձ���ϡ�����ƣ�����������С��ձ�������������Ͳ����ͷ�ιܣ�
�ʴ�Ϊ��D��
��2��������������Һ������������Ϊ�����ӣ���������ԭΪ�����ӣ�ͬʱ����ˮ����Ӧ���ӷ���ʽΪ��MnO4-+5Fe2++8H+=5Fe3++Mn2++4H2O��
�ʴ�Ϊ��MnO4-+5Fe2++8H+=5Fe3++Mn2++4H2O��
��3����������Ƿ���ȫ������ȡ������Һ���μ�KSCN��Һ������ɫ�仯˵��������ȫ����ZnO����pH��ʹFe3+ת��Ϊ����������������ȥ���������ӳ�����ȫ��ͭ���ӿ�ʼ����pH��֪��������ҺPHΪ4.1��4.7��
�ʴ�Ϊ��ȡ������Һ���μ�KSCN��Һ������ɫ�仯��ȡ������Һ���μ�KSCN��Һ������ɫ�仯��4.1��4.7��
��4������III�м���Zn�۽�һ��������ҺpH������ԭͭ����Ϊͭ���ʣ����˳�ȥ��
�ʴ�Ϊ����ȥ��Һ�е�Cu2+��
��5������IV��ʹ����NH4HCO3��Һ�ܴٽ�Zn2+ת��Ϊ���������¶Ȳ��˹��ߣ���ԭ�������̼����������ֽ⣬
�ʴ�Ϊ��NH4HCO3�ֽ����ʧ��
��6���ζ��յ�ʱ��Һ��ɫ�ɳ�ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ��
����H++OH-=H2O��֪�����ᷴӦ��ʣ�����������ʵ���Ϊ0.500mol/L��0.0120L=0.006mol��ZnO��Ӧ�����������ʵ���Ϊ=0.0150L��1.000mol/L��2-0.006mol=0.024mol����ZnO+2H+=Zn2++H2O��֪��ZnO������Ϊ0.024mol��$\frac{1}{2}$��81g/mol=0.972g������Ʒ����Ϊ$\frac{0.972g}{1.000g}$��100%=97.2%��
�ʴ�Ϊ����Һ��ɫ�ɳ�ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ��97.2%��
��7����Ϻ���Һ��c��S2��=3.0��10-3mol•L-1��$\frac{1}{2}$=1.5��10-3mol•L-1������Ksp��CuS��=c��Cu2+����c��S2������֪����ʱ�����Һ��c��Cu2+��=$\frac{6.0��1{0}^{-36}}{1.5��1{0}^{-3}}$mol/L=4.0��10-33mol•L-1������ϡ�Ͷ��ɣ���֪����ͭ��ҺŨ�ȵ��ڻ����Һ��ͭ����Ũ��2����������ͭ��Ũ��Ӧ����Ϊ8.0��10-33mol•L-1��
�ʴ�Ϊ��4.0��10-33mol•L-1��
���� ���⿼�������Ʊ�ʵ�顢�Բ����ķ������ۡ����ʺ����ij������ζ����㡢�ܶȻ��йؼ���ȣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | �ɷǽ���Ԫ���γɵĻ����ﶼ�ǹ��ۻ����� | |
| B�� | �ᡢ�����Ӧ��ֻҪ����1molˮ�ų�����������ͬ | |
| C�� | ԭ��ع���ʱ�������������� | |
| D�� | ij��������ˮ�õ�����Һ���Ե��磬�������һ�������ӻ����� |
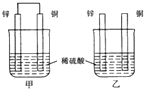
| A�� | ���ձ�����Һ��pH������ | B�� | ����ͭƬ������������ͭƬ�Ǹ��� | ||
| C�� | ���ձ���ͭƬ����������ݲ��� | D�� | �ס�����Һ������ɫ |

| A�� | ͼ�����еķ�Ӧ��Ϊ������ԭ��Ӧ | |
| B�� | ��Ӧ�٢ھ�Ϊ���ӷ�Ӧ | |
| C�� | ��Ӧ�ڢ۶��ܲ����������Ҳ���������������Al��������ת�Ƶ������ֱ���� | |
| D�� | ��Ӧ�٢ڢ�����������ԭ�� |
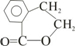
| -C�TC- | ��CH2CH3 ��C2H5 | ��OH | ��CHO | ��COOH | -COO-R |
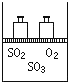 ��ͼ����4mol SO2��2mol O2�����������ɱ�ĵ�ѹ�ܱ������У���һ���¶��·������·�Ӧ2SO2��g��+O2��g��?2SO3��g������H��0���÷�Ӧ�ﵽƽ��״̬ʱ���������������ʵ���Ϊ4.2mol���Իش�
��ͼ����4mol SO2��2mol O2�����������ɱ�ĵ�ѹ�ܱ������У���һ���¶��·������·�Ӧ2SO2��g��+O2��g��?2SO3��g������H��0���÷�Ӧ�ﵽƽ��״̬ʱ���������������ʵ���Ϊ4.2mol���Իش�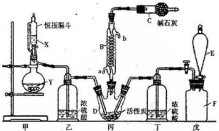

 ��ˮ������С�մ���Һ��Ӧ�Ļ�ѧ����ʽ��
��ˮ������С�մ���Һ��Ӧ�Ļ�ѧ����ʽ��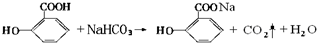 ��
��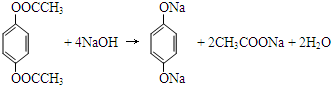 ��
��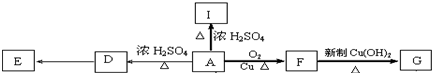 ��
�� E
E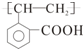 ��
��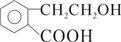 +O2$��_{��}^{Cu}$2
+O2$��_{��}^{Cu}$2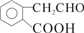 +2H2O
+2H2O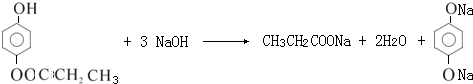 ��
��