��Ŀ����
��һ�ݻ��̶����ܱշ�Ӧ�����м���һ���������ƶ��ĵ��ȵĸ��彫�����ֳɼס��������֣��ֱ��������������淴Ӧ��
�ף�a(g)��b(g)  2c(g) ��H1<0�� �ң� x(g)��3y(g)
2c(g) ��H1<0�� �ң� x(g)��3y(g)  2z(g) ��H2>0
2z(g) ��H2>0
����ס��Ҿ��ﵽ��Ӧƽ������λ�����м䣬Ȼ�������ز��������������������(����)
| A�������������ͨ��������壬c�����ʵ������� |
| B��������������ͨ��z���壬��Ӧ�����¶����� |
| C�������������ͨ��������壬����ƽ�ⲻ�ƶ�������X��Y��ת�������� |
| D��������������ͨ��z���壬y�����ʵ���Ũ������ |
A
�������������A�������������ͨ��������壬���������ƶ�������ƽ�������ƶ��������������м�����ǵ��ȵģ����¼����¶Ƚ��ͣ�ƽ�������ƶ���c�����ʵ���������B��������������ͨ��z���壬ƽ������ȵ������ƶ�����Ӧ�����¶����ߣ���ȷ��C�������������ͨ��������壬����ƽ�ⲻ�ƶ�������ƽ����ѹ���������ƶ���X��Y��ת����������ȷ��D��������������ͨ��z���壬ƽ�������ƶ���y�����ʵ���Ũ��������ȷ��
���㣺���黯ѧƽ���Ӱ�����ء�

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д���ͬ�¶��£��ݻ���ͬ�ļס������������ܱ������о��������·�Ӧ��2SO2(g) + O2(g)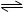 2SO3(g) + 197 kJʵ�����й��������£�
2SO3(g) + 197 kJʵ�����й��������£�
| ������� | ��ʼʱ�����ʵ����ʵ��� / mol | �ﵽƽ��ʱ��ϵ�����ı仯/KJ | ||
| SO2 | O2 | SO3 | ||
| �� | 2 | 1 | 0 | �ų�������Q1 |
| �� | 1.8 | 0.9 | 0.2 | �ų�������Q2 |
�����ж��в���ȷ����
A��197��Q1��Q2
B���ﵽƽ��ʱSO2������������ף���
C���������з�Ӧ��ƽ�ⳣ�����
D������1molSO3(l)ʱ�ų�����������98.5kJ
���淴ӦA(��)��aB(g)  C(g)��2D(g)(aΪ������)����Ӧ�����У���������������ʱ��C�İٷֺ���(C%)���¶�(T)��ѹǿ(P)�Ĺ�ϵ��ͼ��ʾ������˵������ȷ����
C(g)��2D(g)(aΪ������)����Ӧ�����У���������������ʱ��C�İٷֺ���(C%)���¶�(T)��ѹǿ(P)�Ĺ�ϵ��ͼ��ʾ������˵������ȷ����
| A����a��2����AΪҺ̬����� |
| B���÷�Ӧ������ӦΪ���ȷ�Ӧ |
| C��T2��T1��P2��P1 |
| D�������������䣬����B�����ʵ�����ƽ�������ƶ���ƽ�ⳣ��K���� |
���ĸ���ͬ�������н��кϳɰ��ķ�Ӧ��������������ͬʱ���ڲⶨ�Ľ�����ж����ɰ�������������
| A��v (N2)=0��05mol��L��1��s��1 | B��v (H2)=0��3mol��L��1��min��1 |
| C��v (N2)=0��2 mol��L��1��min��1 | D��v (NH3)=0��3 mol��L��1��min��1 |
�����й�˵����ȷ����
| A���ϳɰ���Ӧ��ʹ�ô�����˵���������Դٽ���ƽ�������ɰ��ķ����ƶ� |
| B��Ǧ�����ڷŵ�����У������������ӣ������������� |
| C��100 mL pH��3��HA��Һ��HB��Һ�ֱ���������п��Ӧ��HA��Һ�ų��������࣬˵��HA�����Ա�HB���� |
D����֪I3- I2��I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ� I2��I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ� |
����˵����ȷ���ǣ� ��
| A����H��0����S��0�ķ�Ӧ���¶ȵ�ʱ�����Է����� |
| B��NH4HCO3(s)=NH3(g)��H2O(g)��CO2(g) ��H����185.57 kJ/mol���Է����У�ԭ������ϵ���Է�������Ҷ����ӵķ���ת������� |
| C����Ϊ�ʱ���ر䶼�뷴Ӧ���Է����йأ�����ʱ���ر�����Ե�����Ϊ��Ӧ�Է��Ե��о� |
| D������������������������£�ʹ�ô������Ըı仯ѧ��Ӧ���еķ��� |
ij��ѧ����С���о���������������ʱ���ı�ijһ�����Ի�ѧƽ���Ӱ�죬�õ����±仯���ɣ�ͼ��P��ʾѹǿ��T��ʾ�¶ȣ�n��ʾ���ʵ�������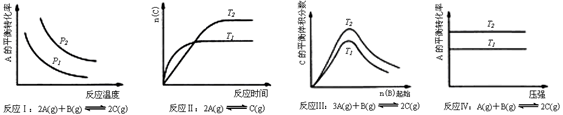
�������Ϲ����жϣ����н�����ȷ����
| A����Ӧ��H��0��p2��p1 |
| B����Ӧ��H>0��T1��T2 |
| C����Ӧ��H��0��T2��T1�����H��0��T2��T1 |
| D����Ӧ������H��0��T2��T1 |

 pC(g)+qD(g) ��H<0�������������
pC(g)+qD(g) ��H<0�������������