��Ŀ����
����Ŀ�����������ʵ���Һ����CH3COOH ��HCl �� NaHSO4
(1)����c(H+)��Ϊ0.1mol��L-1��������Һ��ˮϡ��100��������c(H+)������________(����ű�ʾ����ͬ)��
(2)��������Һ��c(H+)��Ϊ0.1mol��L-1���ֱ���������Һ��Ͷ���С��״����ͬ��п����һ��ʱ���Ӧ������________________��
(3)���ⶨ���ʵ���Ũ����ͬ��������Һ�ĵ���������С��������������_______________��
(4)����6 g CH3COOH����ˮ�Ƴ�1L��Һ������Һ�����ʵ���Ũ��Ϊ________________�����ⶨ��Һ��c(CH3COO��)Ϊ1.4��10-3mol��L-1�����¶��´���ĵ��볣��K=_____________���������� NaHSO4�����K____________(�������������������������С��)��
���𰸡��� �� �� 0.1mol/L 1.96��10-5 ����
��������
ǿ�������ȫ���룬���������ʵĵ���ƽ�⣻�������������Һ�д��ڵ���ƽ�⣬��ˮϡ�ͺ�ƽ�������ƶ������Ӹ�������������Ũ�ȼ�С����Һ�ĵ���������Һ������Ũ�ȵĴ�С����������������йأ�������ʵĵ���ƽ�ⳣ��=�ѵ����������Ũ����֮������ʣ��������ʷ���Ũ�ȣ������Ϸ������н��
��1������ͬ������Ũ��ʱ���������ʵ���Ũ��������Һ�Ǵ�����Һ��ϡ��100������ϡ�����У�����̶�����H+Ũ�ȴ�����������������е�H+Ũ�ȡ�
��2��c(H+)��Ϊ0.1mol��L-1��������Һ������пͶ������H+��HCl��NaHSO4��ǿ����ʣ� H+�����ģ�c(H+)Ѹ�ټ�С����������Һ���е���ƽ�⣬c(H+)��С������ƽ�������ƶ�������̶�Խ��Խ��ʹ��c(H+)ʼ�ձ�HCl��NaHSO4��Һ�д�������Ũ�ȴ�Ӧ���ʿ졣
��3����Һ�ĵ�����������Һ�����ӵ�Ũ�ȡ����������ĵ�����йأ����Ӵ��ĵ����Խ�ࡢ����Ũ��Խ����Һ�ĵ�������Խǿ��NaHSO4����������ǿ��
��4�������Ũ��= =0.1mol/L��c(CH3COO��)=c(H+)=1.4��10-3mol��L-1��c(CH3COOH)=0.1 mol/L��1.4��10-3mol��L-1��0.1 mol/L��K=
=0.1mol/L��c(CH3COO��)=c(H+)=1.4��10-3mol��L-1��c(CH3COOH)=0.1 mol/L��1.4��10-3mol��L-1��0.1 mol/L��K=![]() = 1.96��10-5��Kֻ���¶�Ӱ�죬�¶Ȳ��䣬K���䡣
= 1.96��10-5��Kֻ���¶�Ӱ�죬�¶Ȳ��䣬K���䡣

����Ŀ��FeCl2��һ�ֳ��õĻ�ԭ����ýȾ����ij��ѧʵ��С����ʵ���������������ַ������Ʊ���ˮFeCl2���й����ʵ��������£�
| C6H5Cl(�ȱ��� | C6H4Cl2(���ȱ��� | FeCl3 | FeCl2 |
�ܽ��� | ������ˮ�������ڱ����Ҵ� | ������C6H5Cl��C6H4Cl2������ �������Ҵ�������ˮ | ||
�۵�/�� | -45 | 53 | ������ | |
�е�/�� | 132 | 173 | ||
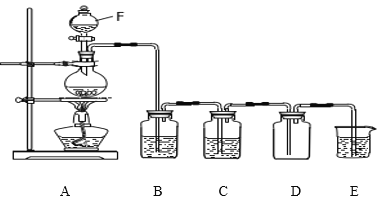
(1)��H2��ԭ��ˮFeCl3��ȡFeCl2���й�װ�����£�

��H2��ԭ��ˮFeCl3��ȡFeCl2�Ļ�ѧ����ʽΪ_____________��
�ڰ����������ҵķ�����������������˳��Ϊ_________������ĸ��װ�ÿɶ��ʹ�ã���C��ʢ�ŵ��Լ���_____________��
�۸��Ʊ�װ�õ�ȱ��Ϊ________________��
(2)���÷�Ӧ2FeCl3+C6H5Cl��2FeCl2+C6H4Cl2+HCl������ȡ��ˮFeCl2���ⶨFeCl3��ת���ʡ�����ͼװ�ã���������ƿ�з���32.5g��ˮ�Ȼ����������ȱ������Ʒ�Ӧ�¶���һ����Χ����3h����ȴ�������ᴿ�õ��ֲ�Ʒ��

������a��������__________��
�ڷ�Ӧ��������ȴʵ��װ��A����������ƿ�����ʵ����������ˡ�ϴ�ӡ�����õ��ֲ�Ʒ��ϴ�����õ��Լ�������____��������Һ��C6H5C1�IJ���������______��
�۷�Ӧ����ƿ����Һ���250mL����ȡ25.00mL������Һ����0.40mol/LNaOH��Һ�ζ����յ�ʱ����NaOH��ҺΪ19.60 mL�����Ȼ�����ת����Ϊ__________��
��Ϊ�˼���ʵ��������ȡ��ˮFeCl2������Ӧ��ȡ�Ĵ�ʩ�У�________��д��һ�㼴�ɣ���
����Ŀ��������25��ʱ�������ܵ���ʵ��ܽ�ȣ�
���ܵ���� | Mg(OH)2 | Cu(OH)2 | Fe(OH)2 | Fe(OH)3 |
�ܽ��/g | 9��10��4 | 1.7��10��6 | 1.5��10��4 | 3.0��10��9 |
������������ᴿ�У����������ܵ���ʵ��ܽ�ƽ��ԭ����ȥijЩ���ӣ��磺
��Ϊ�˳�ȥ�Ȼ���е�����Fe3�����Ƚ����������ˮ���ټ���һ�������Լ���Ӧ�����˽ᾧ���ɣ�
��Ϊ�˳�ȥ�Ȼ�þ�����е�����Fe3�����Ƚ����������ˮ������������������þ����ַ�Ӧ�����˽ᾧ���ɣ�
��Ϊ�˳�ȥ����ͭ�����е�����Fe2�����Ƚ����������ˮ������һ������H2O2����Fe2��������Fe3����������Һ��pH��4�����˽ᾧ���ɣ�
��ش��������⣺
��1�������������ӷ������ܹ��ﵽ�ܺõ�Ч����Fe2����Fe3������ת��Ϊ____________(�ѧʽ)����ȥ��
��2�����м�����Լ�Ӧ��ѡ��________Ϊ�ˣ���ԭ����__________��
��3�����г�ȥFe3�����������ܷ�Ӧ�����ӷ���ʽΪ______________________________��
��4�������뷽������ص������У���ȷ����______(����ĸ)��
A��H2O2����ɫ�������������������в��������ʣ���������Ⱦ
B����Fe2������ΪFe3������Ҫԭ����Fe(OH)2������Fe(OH)3�������ѹ���
C��������ҺpH��4��ѡ����Լ���������ͭ���ʽ̼��ͭ������ͭ
D��Cu2�����Դ���������pH��4����Һ��
E����pH��4����Һ��Fe3��һ�����ܴ�������