��Ŀ����
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������A��Dͬ���壬C��Eͬ���壬D��E��Fͬ���ڣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C�γɵ���������ȵķ��ӣ���A��C�γɵĻ����ﳣ����ΪҺ̬��A�ֱܷ���E��F�γɵ���������ȵ�������ӣ�
��ش��������⣺
��1��E��F��̬�⻯����ȶ���Ϊ
��2��E��F���γ�E2F2�Ļ���������ʽΪ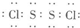
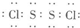 ���侧������Ϊ
���侧������Ϊ
��3��C��D�γɵ�һ�ֻ���������D��E�γɵĻ���������Һ�з���������ԭ��Ӧ�������ӷ���ʽΪ��
��4��A��C��E����Ԫ���γɵ�һ�ֳ���������H����Ũ��Һ�ڼ��������¿���a gͭ��Ӧ����ԭ��H�����ʵ���Ϊ
mol
mol��
��5��E��һ�ֳ���������Ϊ������Ⱦ�ʵ���ҿ�������D������������ˮ���������գ����������ɵ�����Һ������Ũ�ȴ�С��ϵΪ��
��6��b g D�����ڴ�����C������ȼ�շų�Q kJ����������ص��Ȼ�ѧ����ʽΪ��
��ش��������⣺
��1��E��F��̬�⻯����ȶ���Ϊ
HCl
HCl
��H2S
H2S
���û�ѧʽ��ʾ������2��E��F���γ�E2F2�Ļ���������ʽΪ
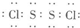
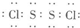
���Ӿ���
���Ӿ���
����3��C��D�γɵ�һ�ֻ���������D��E�γɵĻ���������Һ�з���������ԭ��Ӧ�������ӷ���ʽΪ��
Na2O2+S2-+2H2O=S��+2Na++4OH-
Na2O2+S2-+2H2O=S��+2Na++4OH-
����4��A��C��E����Ԫ���γɵ�һ�ֳ���������H����Ũ��Һ�ڼ��������¿���a gͭ��Ӧ����ԭ��H�����ʵ���Ϊ
| a |
| 64 |
| a |
| 64 |
��5��E��һ�ֳ���������Ϊ������Ⱦ�ʵ���ҿ�������D������������ˮ���������գ����������ɵ�����Һ������Ũ�ȴ�С��ϵΪ��
c��Na+����c��SO32-����c��OH-����c��HSO3-����c��H+��
c��Na+����c��SO32-����c��OH-����c��HSO3-����c��H+��
����6��b g D�����ڴ�����C������ȼ�շų�Q kJ����������ص��Ȼ�ѧ����ʽΪ��
2Na��s��+O2��g��=Na2O2��s����H=-
kJ/mol
| 46Q |
| b |
2Na��s��+O2��g��=Na2O2��s����H=-
kJ/mol
��| 46Q |
| b |
������A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������A��C�γɵĻ����ﳣ����ΪҺ̬��������Ϊˮ����AΪH��CΪO��A��B������������֮����C��������������ȣ�B������������Ϊ5����BΪNԪ�أ�A��Dͬ���壬��DΪNa��C��Eͬ���壬��EΪS��D��E��Fͬ���ڣ�A�ֱܷ���E��F�γɵ���������ȵ�������ӣ���FΪCl��
��1���ǽ�����Խǿ����̬�⻯��Խ�ȶ���
��2��E2F2ΪS2Cl2���Թ��ۼ��γɵķ��ӣ�Ϊ���Ӿ��壻
��3��C��D�γɵ�һ�ֻ�����ΪNa2O2������ǿ�����ԣ�D��E�γɵĻ�����ΪNa2S��
��4��������HΪ���ᣬ�ɵ����غ������㣻
��5������������������NaOH��Һ���������������ƣ�����ˮ����������
��6���������ʵ����뷴Ӧ�ų������������ȼ��Ȼ�ѧ��Ӧ����ʽ����д�����
��1���ǽ�����Խǿ����̬�⻯��Խ�ȶ���
��2��E2F2ΪS2Cl2���Թ��ۼ��γɵķ��ӣ�Ϊ���Ӿ��壻
��3��C��D�γɵ�һ�ֻ�����ΪNa2O2������ǿ�����ԣ�D��E�γɵĻ�����ΪNa2S��
��4��������HΪ���ᣬ�ɵ����غ������㣻
��5������������������NaOH��Һ���������������ƣ�����ˮ����������
��6���������ʵ����뷴Ӧ�ų������������ȼ��Ȼ�ѧ��Ӧ����ʽ����д�����
����⣺A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������A��C�γɵĻ����ﳣ����ΪҺ̬��������Ϊˮ����AΪH��CΪO��A��B������������֮����C��������������ȣ�B������������Ϊ5����BΪNԪ�أ�A��Dͬ���壬��DΪNa��C��Eͬ���壬��EΪS��D��E��Fͬ���ڣ�A�ֱܷ���E��F�γɵ���������ȵ�������ӣ���FΪCl��
��1���ǽ�����Cl��S������̬�⻯����ȶ�ΪHCl��H2S���ʴ�Ϊ��HCl��H2S��
��2��E2F2ΪS2Cl2���Թ��ۼ��γɵķ��ӣ������ʽΪ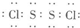 ��������Ϊ���ӣ���Ϊ���Ӿ��壬�ʴ�Ϊ��
��������Ϊ���ӣ���Ϊ���Ӿ��壬�ʴ�Ϊ��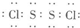 �����Ӿ��壻
�����Ӿ��壻
��3��C��D�γɵ�һ�ֻ�����ΪNa2O2������ǿ�����ԣ�D��E�γɵĻ�����ΪNa2S�����߷���������ԭ��Ӧ�������ӷ�ӦΪNa2O2+S2-+2H2O=S��+2Na++4OH-��
�ʴ�Ϊ��Na2O2+S2-+2H2O=S��+2Na++4OH-��
��4��������HΪ���ᣬCu��Ũ���ᷴӦ��Cuʧȥ���ӣ�S�õ����ӣ���S�����ʵ���Ϊx���ɵ����غ��֪��x����6-4��=
����2-0�������x=
mol��
�ʴ�Ϊ��
mol��
��5������������������NaOH��Һ���������������ƣ������������ˮ���Լ��ԣ���ˮ����������У������ӵ�Ũ��Ϊc��Na+����c��SO32-����c��OH-����c��HSO3-����c��H+����
�ʴ�Ϊ��c��Na+����c��SO32-����c��OH-����c��HSO3-����c��H+����
��6��bgNaȼ�����ɹ������Ʒų�������ΪQkJ����46gNaȼ�շų�������Ϊ
kJ�����Ȼ�ѧ��Ӧ����ʽΪ2Na��s��+O2��g��=Na2O2��s����H=-
kJ/mol��
�ʴ�Ϊ��2Na��s��+O2��g��=Na2O2��s����H=-
kJ/mol��
��1���ǽ�����Cl��S������̬�⻯����ȶ�ΪHCl��H2S���ʴ�Ϊ��HCl��H2S��
��2��E2F2ΪS2Cl2���Թ��ۼ��γɵķ��ӣ������ʽΪ
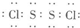 ��������Ϊ���ӣ���Ϊ���Ӿ��壬�ʴ�Ϊ��
��������Ϊ���ӣ���Ϊ���Ӿ��壬�ʴ�Ϊ��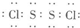 �����Ӿ��壻
�����Ӿ��壻��3��C��D�γɵ�һ�ֻ�����ΪNa2O2������ǿ�����ԣ�D��E�γɵĻ�����ΪNa2S�����߷���������ԭ��Ӧ�������ӷ�ӦΪNa2O2+S2-+2H2O=S��+2Na++4OH-��
�ʴ�Ϊ��Na2O2+S2-+2H2O=S��+2Na++4OH-��
��4��������HΪ���ᣬCu��Ũ���ᷴӦ��Cuʧȥ���ӣ�S�õ����ӣ���S�����ʵ���Ϊx���ɵ����غ��֪��x����6-4��=
| ag |
| 64g/mol |
| a |
| 64 |
�ʴ�Ϊ��
| a |
| 64 |
��5������������������NaOH��Һ���������������ƣ������������ˮ���Լ��ԣ���ˮ����������У������ӵ�Ũ��Ϊc��Na+����c��SO32-����c��OH-����c��HSO3-����c��H+����
�ʴ�Ϊ��c��Na+����c��SO32-����c��OH-����c��HSO3-����c��H+����
��6��bgNaȼ�����ɹ������Ʒų�������ΪQkJ����46gNaȼ�շų�������Ϊ
| 46Q |
| b |
| 46Q |
| b |
�ʴ�Ϊ��2Na��s��+O2��g��=Na2O2��s����H=-
| 46Q |
| b |
���������⿼��λ�á��ṹ�����ʵĹ�ϵ��Ӧ�ã��ۺ��Խ�ǿ������֪ʶ��϶࣬Ԫ�ص��ƶ��ǽ����Ĺؼ�������ϤԪ�ػ����������������ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��






