��Ŀ����
��ѧ�����������ࡰ���ࡱ�����ʡ����������ڿ�����ͨ�����ȶ����ױ���������FeSO4��7H2O����(NH4) 2Fe(SO4)2��6H2O�����FeSO4��7H2O����������������ȶ����࣬���ڷ�����ѧ�������Լ���������FeSO4��7H2O�ڿ���������ʧȥ�ᾧˮ��ΪFeSO4����(NH4) 2Fe(SO4)2.��6H2Oʧȥ�ᾧˮ�������ࡣ
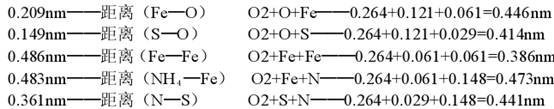
FeSO4��7H2O�ľ�������Ϊa=1.407nm��b=0.6503nm��c=1.104nm����=��=90.00�㣬��=105.57���ܶ�Ϊ1.898g/cm3����ˮFeSO4�����������Fe��O����Ϊ0.212nm��S��O����Ϊ0.151nm��Fe��S����Ϊ0.475nm��Fe��Fe����Ϊ0.769nm��(NH4) 2Fe(SO4)2��6H2O�ľ�������Ϊa=0.932nm��b=1.265nm��c=0.624nm����=��=90.00�㣬��=106.80�㣬�ܶ�Ϊ1.864g/cm3�������Fe��O����0.209nm��S��O����Ϊ0.149nm��Fe��Fe����Ϊ0.486nm��NH4+��Fe����Ϊ0.483nm��N��S����Ϊ0.361nm
�š�����һ��FeSO4��7H2O������һ��(NH4) 2Fe(SO4)2��6H2O�����ֱ�����ԭ�Ӹ���
�ơ����������Ľṹ���϶��Сֱ��Ӱ�컯ѧ��Ӧ���е����ƣ���ͨ������˵��
FeSO4��7H2O��(NH4) 2Fe(SO4)2.��6H2O���ױ�����
��֪��O�Ĺ��۰뾶Ϊ0.66nm���ھ�����+2�۵�Fe�뾶Ϊ0.061nm����2�۵�O�뾶Ϊ0.121nm��+6�۵�S�뾶Ϊ0.029nm����3�۵�N�뾶Ϊ0.148nm
�ǡ�FeSO4��7H2O�ڳ�ʪ�Ŀ����б������IJ���ΪFe(OH)SO4��3H2O��д����Ӧ�Ļ�ѧ����ʽ
_____________________________________________________________
�ȡ���ˮ��Һ��(NH4) 2Fe(SO4)2��6H2O��FeSO4��7H2O�ȶ����൱���������ԭ��
FeSO4��7H2O�ľ�������Ϊa=1.407nm��b=0.6503nm��c=1.104nm����=��=90.00�㣬��=105.57���ܶ�Ϊ1.898g/cm3����ˮFeSO4�����������Fe��O����Ϊ0.212nm��S��O����Ϊ0.151nm��Fe��S����Ϊ0.475nm��Fe��Fe����Ϊ0.769nm��(NH4) 2Fe(SO4)2��6H2O�ľ�������Ϊa=0.932nm��b=1.265nm��c=0.624nm����=��=90.00�㣬��=106.80�㣬�ܶ�Ϊ1.864g/cm3�������Fe��O����0.209nm��S��O����Ϊ0.149nm��Fe��Fe����Ϊ0.486nm��NH4+��Fe����Ϊ0.483nm��N��S����Ϊ0.361nm
�š�����һ��FeSO4��7H2O������һ��(NH4) 2Fe(SO4)2��6H2O�����ֱ�����ԭ�Ӹ���
�ơ����������Ľṹ���϶��Сֱ��Ӱ�컯ѧ��Ӧ���е����ƣ���ͨ������˵��
FeSO4��7H2O��(NH4) 2Fe(SO4)2.��6H2O���ױ�����
��֪��O�Ĺ��۰뾶Ϊ0.66nm���ھ�����+2�۵�Fe�뾶Ϊ0.061nm����2�۵�O�뾶Ϊ0.121nm��+6�۵�S�뾶Ϊ0.029nm����3�۵�N�뾶Ϊ0.148nm
�ǡ�FeSO4��7H2O�ڳ�ʪ�Ŀ����б������IJ���ΪFe(OH)SO4��3H2O��д����Ӧ�Ļ�ѧ����ʽ
_____________________________________________________________
�ȡ���ˮ��Һ��(NH4) 2Fe(SO4)2��6H2O��FeSO4��7H2O�ȶ����൱���������ԭ��
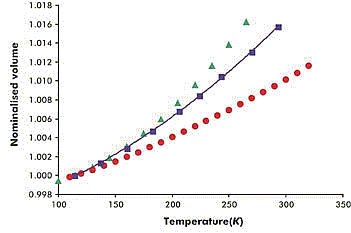
�š�������ã�FeSO4��7H2O ��V=abcsin��,Z=dVNA/M=4��(1��)��ԭ�Ӹ���Ϊ108 ��1�֣�
(NH4) 2Fe(SO4)2��6H2O��V=abcsin��,Z=dVNA/M=2��(1��)��ԭ�Ӹ���Ϊ 78 ��1�֣�
�ơ�O2���ӵ�ֱ����СΪ0.66´4="0.264nm " ��1�֣�
Ҫ��һ��FeSO4.7H2O��ʧˮΪFeSO4��FeSO4������

֪������������ɢ��Fe,S��Fe,Feԭ��֮��Ŀ�϶������Ӧ ��2�֣�
Ҫ�����(NH4) 2Fe(SO4)2��6H2O��������
(NH4) 2Fe(SO4)2��6H2O����ԭ�����и��ӽ��ܣ���FeSO4��Ƚϣ�������������ɢ�뾧���϶�� ��2�֣�
�ǡ�4FeSO4��7H2O+ O2 ="==" 4Fe(OH)SO4��3H2O + 14H2O ��1�֣�
�ȡ��ܽ�����Fe2+������ʽ���ڣ�ˮ��Һ��Fe2+�Ļ�ԭ�Դ�Сȡ������Һ�����ԣ�(NH4) 2Fe(SO4)2��6H2O��FeSO4��7H2Oˮ��Һ���������Ӷ����ȶ������� ��1�֣���˼�Լ��ɣ�
(NH4) 2Fe(SO4)2��6H2O��V=abcsin��,Z=dVNA/M=2��(1��)��ԭ�Ӹ���Ϊ 78 ��1�֣�
�ơ�O2���ӵ�ֱ����СΪ0.66´4="0.264nm " ��1�֣�
Ҫ��һ��FeSO4.7H2O��ʧˮΪFeSO4��FeSO4������

֪������������ɢ��Fe,S��Fe,Feԭ��֮��Ŀ�϶������Ӧ ��2�֣�
Ҫ�����(NH4) 2Fe(SO4)2��6H2O��������

(NH4) 2Fe(SO4)2��6H2O����ԭ�����и��ӽ��ܣ���FeSO4��Ƚϣ�������������ɢ�뾧���϶�� ��2�֣�
�ǡ�4FeSO4��7H2O+ O2 ="==" 4Fe(OH)SO4��3H2O + 14H2O ��1�֣�
�ȡ��ܽ�����Fe2+������ʽ���ڣ�ˮ��Һ��Fe2+�Ļ�ԭ�Դ�Сȡ������Һ�����ԣ�(NH4) 2Fe(SO4)2��6H2O��FeSO4��7H2Oˮ��Һ���������Ӷ����ȶ������� ��1�֣���˼�Լ��ɣ�
�ŴӾ�������������FeSO4��7H2O������ (NH4) 2Fe(SO4)2��6H2O������Ϊ��б��ϵ����˾����ܶȵļ��㹫ʽ���ǣ�

����V��abcsin��
�������ݣ��ɷֱ���ø������еĽṹ��Ԫ����z��1 molFeSO4��7H2O�й���27molԭ�ӣ�1 mol(NH4) 2Fe(SO4)2��6H2O�й���39 molԭ�ӣ��Ӷ���ø������е�ԭ������
�Ƹ�����ʾ�����ھ��������Ľṹ���϶��Сֱ��Ӱ�컯ѧ��Ӧ���е����ƣ���˱���ĺ������ڼ����Fe2+��Χ�Ŀ�϶�Ĵ�С������ÿ�϶�Ĵ�С����O2(264pm)ͨ���Ļ���������ʱ�������������ǿ��
�Ǹ÷�Ӧ���ܸ�д�����ӷ�Ӧ����ʽ����������ƽ��ʱ����Ȼ����������ԭ����ʽ��ƽ�ļ��ɺͷ���������ƽ��������£�
4FeSO4��7H2O+ O2 ="==" 4Fe(OH)SO4��3H2O + 14H2O
�ȴӢ��еļ����֪�����ǵľ�����������ԭ�������ʴ��ڲ������Ϊ�����п�϶�Ĵ�С���ڲ�𡣵�������Һ�У����Ƕ���ǿ����ʣ���ɵ��룬����ˮ��Fe(��)����Fe2+�Ļ�ԭ�Ը����ӵ�Ũ�ȣ���������������ǿ�����Լ���Һ������Եȣ�����Щ�������������ǵľ���ṹû�й�ϵ�������ˮ��Һ��(NH4) 2Fe(SO4)2��6H2O��FeSO4��7H2O�ȶ����൱��

����V��abcsin��
�������ݣ��ɷֱ���ø������еĽṹ��Ԫ����z��1 molFeSO4��7H2O�й���27molԭ�ӣ�1 mol(NH4) 2Fe(SO4)2��6H2O�й���39 molԭ�ӣ��Ӷ���ø������е�ԭ������
�Ƹ�����ʾ�����ھ��������Ľṹ���϶��Сֱ��Ӱ�컯ѧ��Ӧ���е����ƣ���˱���ĺ������ڼ����Fe2+��Χ�Ŀ�϶�Ĵ�С������ÿ�϶�Ĵ�С����O2(264pm)ͨ���Ļ���������ʱ�������������ǿ��
�Ǹ÷�Ӧ���ܸ�д�����ӷ�Ӧ����ʽ����������ƽ��ʱ����Ȼ����������ԭ����ʽ��ƽ�ļ��ɺͷ���������ƽ��������£�
4FeSO4��7H2O+ O2 ="==" 4Fe(OH)SO4��3H2O + 14H2O
�ȴӢ��еļ����֪�����ǵľ�����������ԭ�������ʴ��ڲ������Ϊ�����п�϶�Ĵ�С���ڲ�𡣵�������Һ�У����Ƕ���ǿ����ʣ���ɵ��룬����ˮ��Fe(��)����Fe2+�Ļ�ԭ�Ը����ӵ�Ũ�ȣ���������������ǿ�����Լ���Һ������Եȣ�����Щ�������������ǵľ���ṹû�й�ϵ�������ˮ��Һ��(NH4) 2Fe(SO4)2��6H2O��FeSO4��7H2O�ȶ����൱��

��ϰ��ϵ�д�
�����Ŀ