��Ŀ����
����Ŀ�������£�0.1 mol��L��1��ij��Ԫ��H2A��Һ�У����ܴ��ڵĺ�A����(H2A��HA����A2��)�����ʵ���������pH�仯�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ� ��
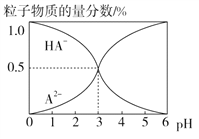
A. pH��5ʱ����NaHA��Na2A�Ļ����Һ�У�c(HA��)��c(A2��)��1��100
B. H2A�ĵ��뷽��ʽ��H2A![]() H����HA��
H����HA��
C. �����ʵ���Ũ�ȵ�NaHA��Na2A��Һ�������ϣ�����Ũ�ȴ�С��ϵΪc(Na��)>c(HA��)>c(A2��)
D. Na2A��Һ�ش���c(OH��)��c(H��)��c(HA��)��2c(H2A)��������Ũ�Ⱦ�����0
���𰸡�A
�������������������ͼ���֪�������£�0.1 mol��L��1��ij��Ԫ��H2A��Һ�У�HA�������ʵ�������Ϊ1.0��˵��H2A��һ����������ȫ���������pH����HA�������ʵ����������ϼ�С��A2�������ʵ�����������������pH=3ʱ��HA����A2�������ʵ��Ƿ�����ͬ����Ϊ0.5. A. pH=3ʱ��HA����A2�������ʵ��Ƿ�����ͬ������![]() ������pH��5ʱ��
������pH��5ʱ�� ![]() ����
����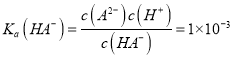 ��֪����NaHA��Na2A�Ļ����Һ����c(HA��)��c(A2��)��1��100��A��ȷ��B. H2A��ǿ�ᣬ����뷽��ʽΪH2A=H����HA����B����ȷ��C. ��ͼ����Ϣ��֪�������ʵ���Ũ�ȵ�NaHA��Na2A��Һ�������ϣ���Һ�����ԣ���HA���ĵ���̶ȴ���A2����ˮ��̶ȣ�����Ũ�ȴ�С��ϵΪc(Na��) >c(A2��) >c(HA��)��C����ȷ��D. Na2A��Һ��������A2��ˮ������HA�����������غ��֪��c(H��)��c(OH��)��c(HA��)��H2AŨ��Ϊ0��D����ȷ������ѡA��
��֪����NaHA��Na2A�Ļ����Һ����c(HA��)��c(A2��)��1��100��A��ȷ��B. H2A��ǿ�ᣬ����뷽��ʽΪH2A=H����HA����B����ȷ��C. ��ͼ����Ϣ��֪�������ʵ���Ũ�ȵ�NaHA��Na2A��Һ�������ϣ���Һ�����ԣ���HA���ĵ���̶ȴ���A2����ˮ��̶ȣ�����Ũ�ȴ�С��ϵΪc(Na��) >c(A2��) >c(HA��)��C����ȷ��D. Na2A��Һ��������A2��ˮ������HA�����������غ��֪��c(H��)��c(OH��)��c(HA��)��H2AŨ��Ϊ0��D����ȷ������ѡA��