��Ŀ����
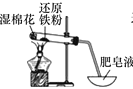
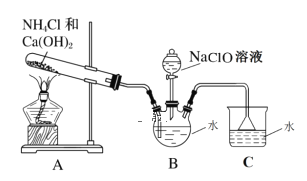
����Ŀ���£�N2H4����һ����Ҫ�Ĺ�ҵ��Ʒ�����ϱ��������ʹ���������Һ��Ӧ�������£����м�ǿ�Ļ�ԭ�ԡ�������ͼװ����ȡ�£�
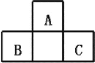
��1��д���µĵ���ʽ__________��д���������ᷴӦ�������ɵ��εĻ�ѧʽ_______��
��2��װ��A�з�Ӧ�Ļ�ѧ����ʽ_____��
��3��ʵ��ʱ���ȵ�ȼA���ľƾ��ƣ�һ��ʱ�������B��������ƿ�еμ�NaClO��Һ���μ�NaClO��Һʱ���ܹ��졢�����ԭ��___________��
��4����ʵ�鰲ȫ�ԽǶ�ָ����ʵ��װ���д��ڵ�ȱ��_______��
��5��ȷ��ȡ20.00mL������Һ�����������̼�����ƣ���0.1000mol/L�ı���Һ���еζ����ζ��յ�ʱ������V0mL���ڴ˹�����N2H4��N2������ʵ���ѡ��______��ָʾ��������Һ���µ�Ũ��Ϊ______mol/L���ú�V0�Ĵ���ʽ���������
���𰸡�![]() N2H6SO4����N2H5��2SO4 ��N2H6��HSO4��2 2NH4Cl+Ca(OH)2
N2H6SO4����N2H5��2SO4 ��N2H6��HSO4��2 2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O ��ֹNaClO������ A��B��������װ�ã���ʹAװ���в�����ը�� ������Һ V0/400
CaCl2+2NH3��+2H2O ��ֹNaClO������ A��B��������װ�ã���ʹAװ���в�����ը�� ������Һ V0/400
��������
(1)�µķ���ʽΪN2H4�����Կ��ɰ��������е�һ����ԭ�ӱ�����ȡ���������Լ��ԣ��¿��Կ��ɶ�Ԫ��ݴ˷������
(2)װ��A���Ʊ�������д��Ӧ�ķ���ʽ��
(3)�������м�ǿ�Ļ�ԭ�Է������
(4)������������ˮ����װ�ú��������������ݴ˽��
(5)���ݱ���Һѡ��ָʾ�����ڴ˹�����N2H4��N2��I2��I-�����ݵ�ʧ�����غ㣬��N2H4��2I2���ݴ˷������㡣
(1)�µķ���ʽΪN2H4������ʽΪ![]() ���¿��Կ��ɰ��������е�һ����ԭ�ӱ�����ȡ���������Լ��ԣ��¿��Կ��ɶ�Ԫ������ᷴӦ�������ɵ�����N2H6SO4��(N2H5)2SO4 ��N2H6(HSO4)2���ʴ�Ϊ��
���¿��Կ��ɰ��������е�һ����ԭ�ӱ�����ȡ���������Լ��ԣ��¿��Կ��ɶ�Ԫ������ᷴӦ�������ɵ�����N2H6SO4��(N2H5)2SO4 ��N2H6(HSO4)2���ʴ�Ϊ��![]() ��N2H6SO4��(N2H5)2SO4 ��N2H6(HSO4)2��
��N2H6SO4��(N2H5)2SO4 ��N2H6(HSO4)2��
(2)����ͼʾ��װ��A�����ɰ����ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O���ʴ�Ϊ��2NH4Cl+Ca(OH)2
CaCl2+2NH3��+2H2O���ʴ�Ϊ��2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
(3)ʵ��ʱ���ȵ�ȼA���ľƾ��ƣ�һ��ʱ�������B��������ƿ�еμ�NaClO��Һ�����м�ǿ�Ļ�ԭ�ԣ����������ƾ���ǿ�����ԣ���˵μ�NaClO��Һʱ���ܹ��졢���࣬��ֹNaClO�����£��ʴ�Ϊ����ֹNaClO�����£�
(4)������������ˮ����װ�ú�������������Ӧ����A��B�����ӷ�����װ�ã��ʴ�Ϊ��A��B��������װ�ã���ʹAװ���в�����ը�ѣ�
(5)ʹ�ñ���Һ���еζ�������ѡ�õ�����Һ��ָʾ�������������һ�ε�Һ�����ױ����ɫ���Ұ�����ڲ���ɫ��������ﵽ�˵ζ��յ㣻�ڴ˹�����N2H4��N2��I2��I-�����ݵ�ʧ�����غ㣬��N2H4��2I2�����ĵ�����ʵ���=0.1000mol/L��V0mL����20.00mL������Һ�к����µ����ʵ���=![]() ��0.1000mol/L��V0mL������µ�Ũ��Ϊ
��0.1000mol/L��V0mL������µ�Ũ��Ϊ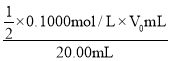 =
=![]() mol/L���ʴ�Ϊ��������Һ��
mol/L���ʴ�Ϊ��������Һ��![]() ��
��

 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�