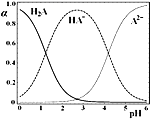
��Ŀ����
����Ŀ��ij100mL���Һ�У�HNO3��H2SO4�����ʵ���Ũ�ȷֱ�Ϊ0.1mol/L��0.4mol/L����û��Һ�м���1.92gͭ�ۣ�����ʹ��Ӧ������ȫ������˵����ȷ����(���Է�Ӧǰ����Һ����仯)�� ��
A.������Һ��c(Cu2+)=0.225mol/L
B.������Һ��c(H+)=0.5mol/L
C.���������ڱ�״���µ����Ϊ0.448L
D.��Ӧ��ת��0.06mol�ĵ���
���𰸡�B
��������
n(Cu)=![]() =0.03mol����Һ��n(H+)=0.1mol/L��0.1L+0.4mol/L��0.1L��2=0.09mol��n(NO3)=0.1mol/L��0.1L=0.01mol��������Һ����ͭ�ۺ�����Ӧ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O���������ӷ�Ӧ����ʽ��֪NO3�������㣬��Ӧ��ͭ�����ʵ���Ϊ0.015mol�����ĵ������ӵ����ʵ���Ϊ0.04mol��
=0.03mol����Һ��n(H+)=0.1mol/L��0.1L+0.4mol/L��0.1L��2=0.09mol��n(NO3)=0.1mol/L��0.1L=0.01mol��������Һ����ͭ�ۺ�����Ӧ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O���������ӷ�Ӧ����ʽ��֪NO3�������㣬��Ӧ��ͭ�����ʵ���Ϊ0.015mol�����ĵ������ӵ����ʵ���Ϊ0.04mol��
A. ��Ӧ��ͭ�����ʵ���Ϊ0.15mol������c(Cu2+)=![]() =0.15mol/L����A����
=0.15mol/L����A����
B. ��Ӧ���ĵ������ӵ����ʵ���Ϊ0.04mol����ʣ���n(H+)=0.09mol-0.04mol=0.05mol����Һ���Ϊ100mL������Ũ��Ϊ0.5mol/L����B��ȷ��
C. �������ӷ���ʽ��֪���ɵ�����n(NO)= n(NO3)=0.01mol����������Ϊ0.224L����C����
D. ��Ӧ������0.01mol NO3����ԭ��NO��ת�Ƶ��ӵ����ʵ���Ϊ0.03mol����D����
�ʴ�ΪB��

����Ŀ����1��25 ��ʱ���Ʊ������������漰���Ȼ�ѧ����ʽ��ƽ�ⳣ�������
�Ȼ�ѧ����ʽ | ƽ�ⳣ�� | |
�� | 2NO2(g)+NaCl(s) | K1 |
�� | 4NO2(g)+2NaCl(s) | K2 |
�� | 2NO(g)+Cl2(g) | K3 |
����¶��£���H3=_______________kJmol-1��K3=_____________����K1��K2��ʾ����
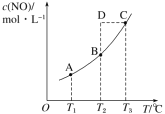
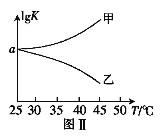
��2��25��ʱ�������Ϊ2L�ĺ����ܱ�������ͨ��0.08 mol NO��0.04 molCl2����������Ӧ�ۣ�����Ӧ��ʼ�����ʱ�¶���ͬ������ѹǿ����ʾ��Ӧ������ѹǿ(p)��ʱ��(t)�ı仯��ͼ��ʵ����ʾ����H3 ___���>����<����=����0��������������ͬ�����ı�ijһ�����������ѹǿ��ʱ��ı仯��ͼ��������ʾ����ı��������_____________����5 minʱ���ٳ���0.08 mol NO��0.04 molCl2�����������ƽ����Է���������_____________�����������С�����䡱����ͼ���Ǽס�����ͬѧ���������Ӧ�۵�ƽ�ⳣ���Ķ���ֵ��lgK�����¶ȵı仯��ϵͼ��������ȷ��������______����ס����ҡ�����aֵΪ__________��25 ��ʱ��÷�Ӧ����ijʱ�̣�NO(g)��Cl2(g)��NOCl(g)��Ũ�ȷֱ�Ϊ0.8��0.1��0.3�����ʱv��_________v�����>����������=����

(3)��300 �桢8 MPa�£���CO2��H2�����ʵ���֮��1��3 ͨ��һ�ܱ������з���CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)�з�Ӧ���ﵽƽ��ʱ�����CO2��ƽ��ת����Ϊ50%����÷�Ӧ�����µ�ƽ�ⳣ��ΪKp��_____(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)��
CH3OH(g)��H2O(g)�з�Ӧ���ﵽƽ��ʱ�����CO2��ƽ��ת����Ϊ50%����÷�Ӧ�����µ�ƽ�ⳣ��ΪKp��_____(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)��
����Ŀ����֪��ӦN2(g)+3H2(g)![]() 2NH3(g)��ij�¶��£�������̶���1L�ܱ������г���1mol N2(g)��3mol H2(g)����ò�ͬʱ�̷�Ӧǰ���ѹǿ��ϵ���±���ʾ��
2NH3(g)��ij�¶��£�������̶���1L�ܱ������г���1mol N2(g)��3mol H2(g)����ò�ͬʱ�̷�Ӧǰ���ѹǿ��ϵ���±���ʾ��
ʱ��/min | 5 | 10 | 15 | 20 | 25 | 30 |
ѹǿ��ֵP��/Pǰ | 0.98 | 0.88 | 0.80 | 0.75 | 0.75 | 0.75 |
(1)0~15min��,��H2��ʾ��ƽ����Ӧ����Ϊv(H2) =___________________mol��L-1��min-1��
(2)�ﵽƽ��ʱN2��ת����Ϊ________�����¶��µ�ƽ�ⳣ��Ϊ___________(������λС��)��
(3)��֪�÷�ӦΪ���ȷ�Ӧ����ͼΪ��ͬ�����·�Ӧ������ʱ��ı仯���(ÿ�ν��ı�һ������)��aʱ�ı������������____________��bʱ�ı������������_______________��

(4)һ�������µ��ܱ������У��÷�Ӧ�ﵽƽ���Ҫ���H2��ת���ʣ����Բ�ȡ�Ĵ�ʩ����_________��
A�����µ�ѹ B��������� C������N2��Ũ�� D������H2��Ũ�� E�������NH3