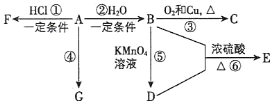
��Ŀ����
����Ŀ���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã�����ѧ�����ش��������⣺
��A | 0 | ||||||||
1 | �� | ��A | ���� | ��A | ��A | ��A | ��A | ��A | |
2 | �� | �� | |||||||
3 | �� | �� | �� | �� | �� | ||||
4 | �� | ||||||||
(1)��Ԫ�ؼ��⻯��ĵ���ʽ��________________________��
(2)�ۡ��ܡ��ݡ���Ԫ�ص����Ӱ뾶�ɴ�С��˳��Ϊ______________��
(3)��Ԫ�ص�����Ϊ_______________���ٳ����ʵ�һ��Ӧ��____________��
(4)��������Ԫ��������������Ӧ��ˮ�����������ǿ����_____________�� д��һ����֤����Ȣ߷ǽ�����ǿ��һ�����ӷ�Ӧ����ʽ___________________��
(5)�����ֻ�����A��B���ɢ٢ۢܢ�����Ԫ����ɡ���A��B��ˮ��Һ���ܷ������ӷ�Ӧ����÷�Ӧ�����ӷ���ʽΪ____________��
(6)����ܵ�ԭ������֮��Ϊ________��
���𰸡� S2��>O2��>Na��>Al3�� �� ��оƬ��̫���ܵ�� HClO4 Cl2+S2����2Cl��+S����Cl2+H2S��2Cl��+S��+2H�� H��+HSO3-=H2O+SO2�� 23
S2��>O2��>Na��>Al3�� �� ��оƬ��̫���ܵ�� HClO4 Cl2+S2����2Cl��+S����Cl2+H2S��2Cl��+S��+2H�� H��+HSO3-=H2O+SO2�� 23
��������
����Ԫ�������ڱ��е�λ�ã�Ԫ�آ١��ڡ��ۡ��ܡ��ݡ��ޡ��ߡ��ࡢ��ֱ���H��N��O��Na��Al��Si��S��Cl��Se��
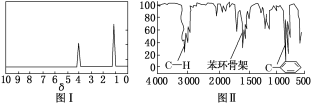
(1)����NԪ�أ����⻯����NH3������ʽ�� ��
��
(2)���Ӳ���Խ�࣬�뾶Խ���Ӳ�����ͬ��������Խ��뾶ԽС��O2-��Na+��Al3+��S2-�뾶�ɴ�С��˳��ΪS2��>O2��>Na��>Al3����
(3)����SiԪ�أ�����Ϊ�裻���ǰ뵼����ϣ�������оƬ��̫���ܵ�أ�
(4)Ԫ�طǽ�����Խǿ��������������Ӧ��ˮ���������Խǿ������������Ӧ��ˮ�����������ǿ����HClO4���������û��������е��������������Դ���S����֤��Cl��S�ǽ�����ǿ�����ӷ�Ӧ����ʽCl2+S2����2Cl��+S����
(5)��H��O��Na��S����Ԫ����ɵĻ�����NaHSO4��NaHSO3��ˮ��Һ���ܷ������ӷ�Ӧ���������ơ���������ˮ����÷�Ӧ�����ӷ���ʽΪH��+HSO3-=H2O+SO2����
(6)����34��SeԪ�ء�����11��NaԪ�أ�ԭ������֮��Ϊ34-11=23��

����Ŀ��NF3�������������ڳ��³�ѹ������ɫ����ζ�����壬�����ӹ�ҵ��һ�������ĵ�����ʴ�����塣�ش��������⣺
��1��NF3�ĵ���ʽΪ______��NԪ�صĻ��ϼ�Ϊ______��
��2��F2��NH3ֱ�ӷ�Ӧ����NF3�Ļ�ѧ����ʽΪ______��
��3��ʵ����ģ�ҵ�����õ������NH4HF2��NH4FHF������ȡNF3������Ϊ��NiΪ�������ϵĺϽ��ں����������������������������ķ�Ӧ��������Ϊ̼�ظ֣�����Һ�ɻ��������á�
�ٵ��ʱNF3��______�����ɣ�����������������______���ѧʽ����
�ڵ����Һ����Ni����Fe��Cu�ĵ��ʼ�NH4HF2�ȣ��ɾ��������̽��л��������ã�
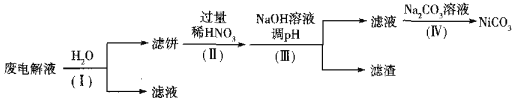
��֪��ʵ�������£����ֽ������ӿ�ʼ�����������ȫ��pH���±�
�������� | Ni2+ | Fe2+ | Cu2+ | Fe3+ |
��ʼ����ʱ��pH | 7.2 | 7.0 | 4.7 | 1.9 |
������ȫʱ��pH | 9.2 | 9.0 | 6.7 | 3.2 |
����I��Ŀ����______��������˱���Ni������������ӷ���ʽΪ______��HNO3�Ļ�ԭ����ΪNO������������pHʱ��������pHӦ���Ƶķ�Χ��______��