��Ŀ����
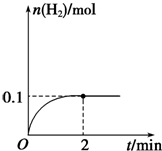
6����һ�ݻ�������ܱ������г���һ����A��B���������·�Ӧ��xA��g��+2B��s��?yC��g����H��0����һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ������AΪ��ɫ���壮��ش��������⣺��1����C��Ũ�ȱ仯��ʾ�÷�Ӧ0��10min�ڵ�ƽ����Ӧ����v��C��=0.04mol/��L•min��
��2������ͼʾ��ȷ��x��y=1��2����x��yΪ��С���������÷�Ӧ�Ļ�ѧ����ʽΪA��g��+2B��s��?2C��g��
��3���Ʋ��10min�������߱仯�ķ�Ӧ���������Ǣܢ�
�ټ�ѹ��������A��Ũ�ȡ�������C�����������¡��ݽ��¡��Ӵ���
��4����ƽ����ƽ�ⳣ��ΪK1��ƽ���ƽ�ⳣ��ΪK2����K1��K2�����������=����������
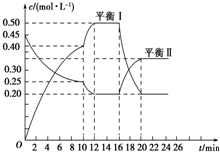
��5��ij�ݻ�������ܱ������У�������������Ϊ�÷�Ӧ��ƽ��״̬���ж�������acd
a������������ɫ���ֲ��䣻 b��v��A������v��C����=x��y
c�������ܶȲ��ٱ仯�� d������������ѹǿ���ٱ仯��
���� ��1������ͼ���֪0��10min��C��Ũ�ȱ仯Ϊ0.40mol/L���ٸ���$v=\frac{��c}{��t}$���㣻
��2������0��10min��A��C�����ʵ���Ũ�ȱ仯�ı�ֵ���ڷ�Ӧ�м�����֮����ã����ݴ���д��ѧ����ʽ��
��3����ͼ��֪����10minʱ����λʱ����A��C�����ʵ���Ũ�ȱ仯���ϴ�Ӧ��������������Ӱ�����ʵ����ط�����
��4����֪��16minʱ�ı���������¶ȣ������¶ȶ�ƽ���Ӱ�������
��5��a��AΪ��ɫ���壬����������ɫ���ֲ��䣬��A��Ũ�Ȳ��䣻
b��v��A������v��C����=x��y������ָ������Ӧ���ʣ�û�з�ӳ���淴Ӧ������Ӧ���ʵĹ�ϵ��
c���÷�Ӧ���������������н��У��ҷ�Ӧǰ�������������غ㣬���Ե������ܶȲ��ٱ仯ʱ��˵�������ʵ�Ũ�Ȳ��ٷ����仯��
d���÷�ӦΪ���������С�ķ�Ӧ��������������ѹǿ���ٱ仯����˵�������ʵ�Ũ�Ȳ��ٷ����仯���ݴ��жϣ�
��� �⣺��1������ͼ���֪0��10min��C��Ũ�ȱ仯Ϊ0.40mol/L������v��C��=$\frac{0.40mol/L}{10min}$=0.04mol/��L•min����
�ʴ�Ϊ��0.04mol/��L•min����
��2��0��10min��������A�����ʵ���Ũ�ȱ仯Ϊ��0.45mol/L-0.25mol/L=0.2mol/L��0��10min��������C�����ʵ���Ũ�ȱ仯Ϊ��0.40mol/L��x��y=0.2mol/L��0.40mol/L=1��2����x��yΪ��С����������÷�Ӧ�Ļ�ѧ����ʽΪA��g��+2B��s��?2C��g����
�ʴ�Ϊ��1��2��A��g��+2B��s��?2C��g����
��3����10minʱ����λʱ����A��C�����ʵ����仯���ϴ�Ӧ��������������Ϊ�����¶Ȼ���ʹ�ô�����
�ʴ�Ϊ���ܢޣ�
��4����֪��16minʱ�ı�������������¶ȣ����÷�Ӧ�Ƿ��ȷ�Ӧ�����������¶�ƽ�����ƣ���ƽ�ⳣ����С������K1��K2��
�ʴ�Ϊ������
��5��a��AΪ��ɫ���壬����������ɫ���ֲ��䣬��A��Ũ�Ȳ��䣬���Է�Ӧ����ƽ��״̬��
b��v��A������v��C����=x��y������ָ������Ӧ���ʣ�û�з�ӳ���淴Ӧ������Ӧ���ʵĹ�ϵ���������жϷ�Ӧ�Ƿ���ƽ��״̬��
c���÷�Ӧ���������������н��У��ҷ�Ӧǰ�������������غ㣬���Ե������ܶȲ��ٱ仯ʱ��˵�������ʵ�Ũ�Ȳ��ٷ����仯�����Է�Ӧ����ƽ��״̬��
d���÷�ӦΪ���������С�ķ�Ӧ��������������ѹǿ���ٱ仯����˵�������ʵ�Ũ�Ȳ��ٷ����仯�����Է�Ӧ����ƽ��״̬��
��ѡacd��
���� ���⿼�黯ѧƽ���Լ���Ӧ���ʵ����⣬��Ŀ�Ѷ��еȣ�ע��ͼ�����߱仯�ķ�����������������Է�Ӧ���ʺ�ƽ���ƶ���Ӱ�죮

| Fe2O3 | CO[ | Fe | CO2 | |
| ��/mol | 1.0 | 1.0 | 1.0 | 1.0 |
| ��/mol | 1.0 | 2.0 | 1.0 | 1.0] |
������˵����ȷ����ad������ĸ����
a���������������ܶȺ㶨ʱ����־��Ӧ�ﵽƽ��״̬
b������Fe2O3�������CO��ת����
c����������CO��ƽ��ת���ʴ����ҵ�ƽ��ת����
d���ס��������У�CO��ƽ��Ũ��֮��Ϊ2��3��
| ��ʱ��/min | ��0 | ��5 | ��10 | ��15 | ��20 | ��25 | ��30 |
| ��c��X��/��mol/L�� | ��0.2 | ��c | ��0.6 | ��0.6 | ��1.0 | ��c1 | ��c1 |
| ��c��Y��/��mol/L�� | ��0.6 | ��c | ��0.4 | ��0.4 | ��0.4 | ��c2 | ��c2 |
��ǰ10min����NO2��ʾ�ķ�Ӧ����Ϊ��20minʱ�ı�������ǣ����´ﵽƽ��ʱ��NO2�İٷֺ���b����ѡ��ǰ��ĸ����
a������ b����С c������ d�����жϣ�
| A�� | ��Ӧǰ����Һ��Cu2+��Ũ�Ȳ�ͬ | |
| B�� | ��[Cu��NH3��4]2+�����У�Cu2+�ṩ�չ����NH3 �����¶Ե��� | |
| C�� | ��Ӧ�����Һ�����Ҵ�����Һû�з����仯 | |
| D�� | �����ܽ����������ɫ���������[Cu��NH3��4]2+ |
| A�� | ������������ȼ�գ�������ɫ�Ļ��棬ƿ�ڳ��ֵġ�������������СҺ�� | |
| B�� | Ư����Һ�е����������ᣬ����ǿ��Ư��Ч�� | |
| C�� | ��������������ȼ�գ�������ɫ���棬���ɰ�ɫ��Na2O���� | |
| D�� | ��˿��������ȼ�գ������غ�ɫ���̣��Ȼ�����ˮ��Һ�ʻ�ɫ |