��Ŀ����
12��ӡ���ɽ�緢��������׳�۵����������������ľ���������ʲƸ������������ð������Σ���ڵײ��Ļ�ɽ���ռ�����ǿ���ȡ������룮��ǿ�������������ԭ�����ᣮij������ͼ��ʾ�Ĺ����������������ش��������⣺
��1��Ϊ������÷�Ӧ�ų����������Ӵ�����Ӧ��װ�Ƚ����������豸���ƣ��������������������ɹܣ�������������SO3��Ũ����ĽӴ������������SO3�����գ�
��2��Ϊʹ��dz��ȼ�գ���������1ͨ��ȼ���ҵ���������50%��Ϊ���SO2ת���ʣ���������2��������Ϊ�Ӵ����ж���������ȫ����ʱ������������2.5��������������������������1��������2�Ŀ��������ӦΪ1.2��1����6��5��������Ӵ�����SO2��ת����Ϊ95%��b���ų���β���ж���������������Ϊ0.41%�����������������������0.2�ƣ�����β���Ĵ����������ð�ˮ���գ�
��3������������Ϊԭ�ϵ�����������ȣ��ù��յ��ص���AC���ɶ�ѡ����
A������������ B�����������ת�������
C�������ķ������� D������Ҫʹ�ô���
��4���������;�dz��㣬��Ӧ����������Щ����BCD��
A������ B��������Լ�������������ơ��ĺϳ�
C��Ǧ���ص����� D��������Ƶ��Ʊ�
��5������ȼ�ϵ�ȼ���Dz���������SO2����Ҫԭ��֮һ����ȼú�м���������ʯ��ʯ������Ч����úȼ��ʱSO2���ŷţ���д������������з�Ӧ�Ļ�ѧ����ʽCaCO3$\frac{\underline{\;����\;}}{\;}$CaO+CO2����2SO2+2CaO+O2=2CaSO4����2SO2+2CaCO3+O2=2CaSO4+2CO2����
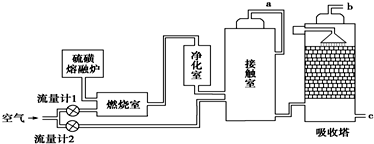
���� ��1��SO2��O2�ķ�ӦΪ���ȷ�Ӧ��Ϊ�˳������������Ӧ��װ�Ƚ����������������������ɹܣ���������������Ũ����ĽӴ��棬������������������գ�
��2���������������Ӧ��װ�Ƚ����������������������ɹܣ���������������Ũ����ĽӴ��棻����SO2�����Ϊx����������1ͨ��ȼ���ҵ���������50%����������1��ͨ�����������Ϊ1.5x����������2��������Ϊ�Ӵ�����SO2��ȫ����ʱ������������2.5������������2��ͨ�����������Ϊ2.5��0.5x=1.25x���ݴ˷������
��3��A��������������������Ҫ����O2��
B��ԭ��ѡ����SO2��ת�����أ�
C����������Ϊԭ�ϲ����ķ����϶࣬����������ͬ��
D����SO2��ȡSO3�Ĺ����ж���Ҫʹ�ô�����
��4��A���������õ���Ϊ������
B��������������к��л��������ȡ��������Ҫ�����ǻ���Ӧ��
C��Ǧ��������Ҫ�õ����������Ǧ��
D��������Ƶ���ȡ��������ҪŨ�������ʯ��
��5��CaCO3���·ֽ�����CO2��CaO��SO2Ϊ������������Ժͼ���������CaO��Ӧ����CaSO3����CaSO3�ױ�����ΪCaSO4��
��� �⣺��1��SO2��O2�ķ�ӦΪ���ȷ�Ӧ��Ϊ�˳������������Ӧ��װ�Ƚ����������������������ɹܣ���������������Ũ����ĽӴ��棬������������������գ�
�ʴ�Ϊ���Ƚ�������ʹŨH2SO4��SO3��ֽӴ���
��2��ȼ�����еķ�ӦΪS+O2$\frac{\underline{\;��ȼ\;}}{\;}$SO2������SO2�����Ϊx����������1��ͨ�����������Ϊ1.5x���Ӵ����еķ�ӦΪ2SO2+O2$\frac{\underline{����}}{��}$2SO3����������2��ͨ�����������Ϊ1.25x��������1��ͨ����������Ϊ7.5x��������2��ͨ����������Ϊ6.25x��������������1��������2�Ŀ��������ӦΪ7.5x��6.25x=6��5��ȼ����ʣ�����6.5x���Ӵ���ʣ�����6.25x-x=5.775x��ʣ��SO2Ϊ0.05x����b��β����SO2���������Ϊ0.41%��SO2Ϊ��������������ü�Һ���簱ˮ�����գ�
�ʴ�Ϊ��6��5��0.41%�� �ð�ˮ���գ�
��3��������������������Ҫ����O2��A����ȷ��ԭ��ѡ����SO2��ת�����أ�B�������������Ϊԭ�ϲ����ķ����϶࣬����������ͬ��C�������SO2��ȡSO3�Ĺ����ж���Ҫʹ�ô�����D�����
��ѡ��A��
��4���������õ���Ϊ������A�����������������к��л��������ȡ��������Ҫ�����ǻ���Ӧ��B����ȷ��Ǧ��������Ҫ�õ����������Ǧ��C����ȷ��������Ƶ���ȡ��������ҪŨ�������ʯ��D����ȷ��
��ѡ��BCD��
��5��CaCO3���·ֽ�����CO2��CaO��SO2Ϊ������������Ժͼ���������CaO��Ӧ����CaSO3����CaSO3�ױ�����ΪCaSO4�����Է�����Ӧ�Ļ�ѧ����ʽΪ��CaCO3$\frac{\underline{\;����\;}}{\;}$CaO+CO2�� SO2+CaO�TCaSO3��2CaSO3+O2�T2CaSO4��
�ʴ�Ϊ��CaCO3$\frac{\underline{\;����\;}}{\;}$CaO+CO2����SO2+CaO�TCaSO3��2CaSO3+O2�T2CaSO4��
���� �����ǶԻ�ѧ�뼼������ҵ�����Ŀ��飬��Ҫѧ��ϸ����������ͼ�и����ʵı仯���н�𣬰��հ�����Ʊ�Ũ����Ĺ������̼��豸�����á������Ļ�ѧ��ӦΪ���Ĺؼ�����Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
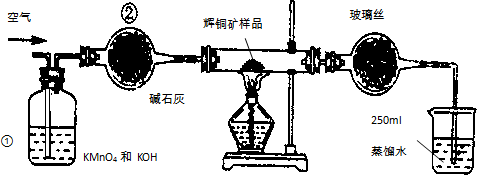
�Ķ��쳵ϵ�д�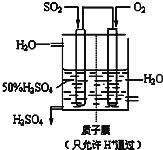 ��ҵ��Ϊ�˲ⶨ��ͭ����Ҫ�ɷ���Cu2S����Cu2S�������������������ͼװ�ã�ʵ��ʱ�����²��������
��ҵ��Ϊ�˲ⶨ��ͭ����Ҫ�ɷ���Cu2S����Cu2S�������������������ͼװ�ã�ʵ��ʱ�����²��������A������ȫ��������ʹ���Ϊ��ͼװ�ã������װ�õ������ԣ�
B����ȡ��ϸ�Ļ�ͭ����Ʒ1.000g��
C���������õ���ƷС�ĵط���Ӳ�ʲ������У�
D����ÿ����1L�����ʹ��������
E����Ӳ�ʲ������еĻ�ͭ����Ʒ���ȵ�һ���¶ȣ�������ӦΪ��Cu2S+O2�TSO2+2Cu��
F����ȡ25.00ml��SO2��ˮ��Һ��250ml��ƿ�У���0.0100mol/L KMnO4����Һ�ζ����յ㣮���������������ظ��ζ�2-3�Σ�
�Իش��������⣺
��1��װ�âٵ������dz�ȥ�����п��ܺ��е�SO2�����壻װ�âڵ������Ǹ������壮
��2���ٶ���ͭ���е���ȫ��ת��ΪSO2������ȫ����ˮ���գ������F����������Ӧ�Ļ�ѧ����ʽΪ2KMnO4+5H2SO3=2MnSO4+K2SO4+2H2SO4+3H2O��
��3��������F�ĵζ���������ʾ�����ͭ����Ʒ��Cu2S������������80%��
| �ζ� ���� | ������Һ�� ���/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.04 | 21.03 |
| 2 | 25.00 | 1.98 | 21.99 |
| 3 | 25.00 | 3.20 | 21.24 |
��5����֪�ڳ�����FeS ��Ksp=6.25��10-18��H2S ������Һ��c��H+����c��S${\;}_{2}^{-}$��֮��������¹�ϵ��c2��H+��•c��S2-��=1.0��10-22���ڸ��¶��£������� FeS Ͷ�����ⱥ����Һ�У���ʹ��Һ�У�Fe2+��Ϊ lmol/L��Ӧ������Һ��c��H+��Ϊ4��10-3mol/L��
��6��ij����������ͼ��ʾװ���õ绯ѧԭ���������ᣬд��ͨ��SO2�ĵ缫�ĵ缫��ӦʽSO2-2e-+2H2O=SO42-+4H+��
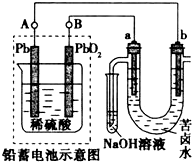 ��Ǧ���ص���±ˮ����C1-��Br-��Na+��Mg2+����װ����ͼ��ʾ��a��bΪʯī�缫��������˵������ȷ���ǣ�������
��Ǧ���ص���±ˮ����C1-��Br-��Na+��Mg2+����װ����ͼ��ʾ��a��bΪʯī�缫��������˵������ȷ���ǣ�������| A�� | Ǧ���طŵ�ʱ���õ��������������С | |
| B�� | Ǧ���س��ʱ��B��Ӧ�����Դ�������� | |
| C�� | ����±ˮʱ��a�缫���ȷŵ����Cl-������Br-����ΪCl-�Ļ�ԭ��ǿ��Br- | |
| D�� | ��b������0.01 mol ����ʱ��Ǧ����������0.02 molH2SO4 |
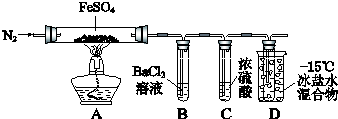
��֪��SO2�۵�-72�棬�е�-10�棻SO3�۵�16.8�棬�е�44.8�森
| ʵ����� | ʵ������ | |
| �� | ͨ��һ��ʱ��N2������ | A�й����Ϊ����ɫ��B���а�ɫ������D�Թ�������ɫҺ�� |
| �� | �ô��л��ǵ�ľ������װ��D�ĵ��ܿ� | ľ����ȼ |
| �� | ��ַ�Ӧ��ֹͣ���ȣ���ȴ��ȡA�й��壬������ | �����ܽ⣬��Һ�ʻ�ɫ |
| �� | ����������Һ����D�Թ��� | ��Һ��Ϊdz��ɫ |
��2��ʵ��۷�Ӧ�����ӷ���ʽ��Fe2O3+6H+=2Fe3++3H2O��
��3���ֽ���̳�����ʹľ����ȼ�������⣬����A�й�����ɫ�仯�Ʋ⣬��һ����SO2���壮��������Ϊ��Fe2O3���ɣ���FeSO4��ֻ��+6��SԪ���������ԣ��ܱ���ԭ�����һ����SO2���ɣ�
��4��ʵ��ܷ�Ӧ�����ӷ���ʽ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��
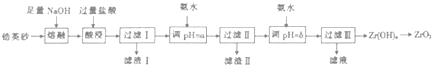
��֪����ZrO2�����ռӦ���ɿ�����ˮ��Na2ZrO2��Na2ZrO2���ᷴӦ����ZrO2+
�ڲ���������ʵ�������¿�ʼ��������ȫ����ʱ��pH���±�•
| ���� | Fe3+ | AP+ | ZrO2+ |
| ��ʼ����pH | 1.9 | 3.3 | 6.2 |
| ���ڳ���pH | 3.2 | 5.2 | 8.0 |
��2������I����Ҫ�ɷֵ�����Ϊ����
��3������ˢ�������Һ�мӰ�ˮ��pH=a����Ŀ���dz�ȥFe3+��Al3+Ȼ������Ӱ�ˮ��pH=b�ķ�Χ��b��8.0
��4������ˢ�������Һ�м���CaCO2��ĩ�����ȣ��ɵ�CO2����һ�����壬�÷�Ӧ�����ӷ���ʽΪ2NH4++CaCO3$\frac{\underline{\;\;��\;\;}}{\;}$Ca2++2NH3��+CO2��+H2O��
| A�� | Na2S��ǿ��ԭ�ԣ�1�����ڳ�ȥ��ˮ�е�Cu2+ ��Hg2+��2�� | |
| B�� | CaCO3�������1��CaCO2��Һ��ͨ��CO2������ɫ������2�� | |
| C�� | ŨHSO4��ǿ�����ԣ�1����ŨH2SO4�����ڸ���SO2��2�� | |
| D�� | Zn���л�ԭ�Ժ͵����ԣ�1������п�̸ɵ�صĸ������ϣ�2�� |

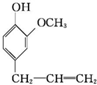 �����������ƿ���ܰ���㾫�Լ����춡��Ӻ������صȣ��ϳɶ�����ӵ�һ��·�����£�
�����������ƿ���ܰ���㾫�Լ����춡��Ӻ������صȣ��ϳɶ�����ӵ�һ��·�����£�
 ��X����±��ԭ�ӣ���
��X����±��ԭ�ӣ��� ��
�� ��
�� ��
��