��Ŀ����
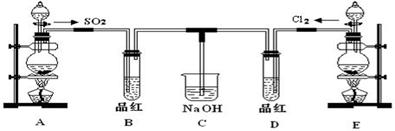
�������ȣ�ClO2����һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ��59�棬�е�Ϊ11.0�棬������ˮ����ҵ���ó�ʪ��KClO3�Ͳ��ᣨH2C2O4����60ʱ��Ӧ�Ƶá�ijѧ����������ͼ��ʾ��װ��ģ����ȡ���ռ�ClO2��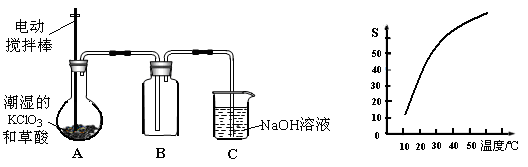
��1��A�з�Ӧ������K2CO3��ClO2��CO2�ȣ���д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2��A���������¶ȿ���װ�ã����ƾ����⣬����Ҫ�IJ����������ձ��� ��BҲ���������¶ȿ���װ�ã�Ӧ���� (ѡ���ˮԡ������ˮԡ��)װ�á�
��3����Ӧ����װ��C�пɵ�NaClO2��Һ����֪NaClO2������Һ�����¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2����������ͼ��ʾ��NaClO2���ܽ�����ߣ��벹���NaClO2��Һ���Ƶ�NaClO2�IJ������裺
�� ���� ����ϴ�ӣ��ܸ��
��4��NaClO2���ʿɷֽ�ΪNaClO3��NaCl��ȡ����������ǰ���NaClO2�����������Һ���ֱ�������FeSO4��Һ��Ӧʱ������Fe2+�����ʵ�����ͬ���ӵ����غ�ĽǶȽ�����ԭ���� ��
ClO2�ܲ��ȶ������������ƣ�������ˮ���յõ�ClO2��Һ��Ϊ�ⶨ������Һ��ClO2�ĺ���������������ʵ�飺
����1��ȷ��ȡClO2��Һ10.00 mL��ϡ�ͳ�100.00 mL��������ȡV1 mL�������뵽��ƿ�У�
����2������������pH��2.0������������KI���壬����Ƭ�̣�
����3���������ָʾ������c mol��L��1 Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��ҺV2 mL������֪2 Na2S2O3 + I2 ��Na2S4O6 + 2NaI��
��5���жϵζ��յ������ ��ԭClO2��Һ��Ũ��Ϊ g / L���ò����е���ĸ����ʽ��ʾ����
��1��2KClO3+ H2C2O4 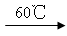 K2CO3+CO2��+2ClO2��+H2O ��2�֣�
K2CO3+CO2��+2ClO2��+H2O ��2�֣�
��2���¶ȼơ�������ˮԡ��������2�֣�
��3�������ᾧ�������ȹ��ˡ�������2�֣�
��4������������ԭ��Ӧ�����е���ת���غ㣬��Ӧ������������Ԫ������Ϊ��1�ۣ�����FeSO4��ʧȥ�ĵ�������ȣ����ĵ�FeSO4����Ҳ��ȡ���������������2�֣�
��5���ӵ����һ��ʱ����Һ����ɫͻȻ����ɫ��Ϊ��ɫ���Ұ���Ӳ��仯��2�֣�����135cV2/V1��2�֣�
���������������1��A�з�Ӧ������K2CO3��ClO2��CO2��,����60�棬����غͲ��ᷴӦ����̼��ء�������̼���������Ⱥ�ˮ����Ӧ����ʽΪ��2KClO3+H2C2O4=K2CO3+CO2��+2ClO2��+H2O;
��2��Ҫ�����¶ȱ���ʹ���¶ȼƲ����¶ȣ���60��ʱ��Ӧ�Ƶã�Ӧ��ˮԡ���ȣ������ձ�����ˮԡ�������������ȵ��۵�ϵͣ�Ϊ�ռ��������ȣ�Ӧ�ڽϵ��¶��½��У�����Ӧ�ò��ñ�ˮԡ���ʴ�Ϊ����ˮԡ�� �ձ�����ˮԡ�������¶ȼƣ�
��3��������ϢNaClO2������Һ�����¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2����������ͼ��ʾ��NaClO2���ܽ�����ߣ������¶������ܽ����������벹���NaClO2��Һ���Ƶ�NaClO2�IJ�������Ϊ�����ᾧ�ͳ��ȹ��ˡ�
��4������������ԭ��Ӧ�����е���ת���غ㣬��Ӧ������������Ԫ������Ϊ��1�ۣ�����FeSO4��ʧȥ�ĵ�������ȣ����ĵ�FeSO4����Ҳ��ȡ�
��5���жϵζ��յ������Ϊ�ζ��յ�ʱ��I2��ȫ��Ӧ����Һ����ɫ��Ϊ��ɫ���ʴ�Ϊ����ɫ��Ϊ��ɫ�Ұ���Ӳ��仯��
��6����ԭClO2��Һ��Ũ��Ϊx��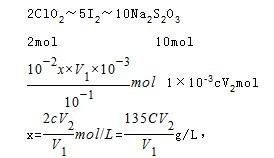
���㣺���⿼����ʵ�鷽������ƣ�ͬʱ����ѧ���������⡢����������������ȷ���ʵ������ǽⱾ��ؼ����ѶȽϴ�

��ϴ�������ǹ辧Ƭ��������Ҫ����֮һ����Ƭ��ѧ��ϴ����ҪĿ���dz�ȥ��Ƭ�������ʣ���ijЩ�л�����Σ�������Si��SiO2�۳��ȣ������õĻ�ѧ��ϴ���иߴ�ˮ���л��ܼ���˫��ˮ��Ũ�ᡢǿ��ȡ�����ȥ����������ͨ����һ��Ũ�ȵ�HF��Һ�����������½���Ƭ����1�������ӡ��������ڹ�Ƭ�����γɽ������ε����棬���ӹ��̫��������ա���������ͨ����NaOH��Na2SiO3�Ȼ����Һ��75��90�淴Ӧ25��35 min��Ч�����á��ش���������
��.��1��д����Ƭ����Ӧ�����ӷ���ʽ ���Ե�������1990�껯ѧ��Seidel�����һ�ֵĵ绯ѧģ�ͣ���ָ��Si��NaOH��Һ�ķ�Ӧ��������Si��OHһ��Ӧ������SiO44һ��Ȼ��SiO44һѸ��ˮ������H4SiO4�����ڴ�ԭ��������Ӧ��������Ϊ ��
��2����У��ѧ��ȤС��ͬѧ��Ϊ��֤Seidel�������Ƿ���ȷ���������ʵ�飺
| | ʵ����ʵ |
| ��ʵһ | ˮ������600��ʱ��ʹ��ĩ״�軺���������ų������� |
| ��ʵ�� | ʢ���ڲ���ʯӢ�����еĴ�ˮ��ʱ��Է�ĩ״��ԭ����ʴ���á� |
| ��ʵ�� | ��ͨ���������е�ˮ�����дӲ������ܳ������ļ���ʹ��ĩ״�������л����ܽ⡣ |
| ��ʵ�� | ��Ұ�����ýϸ߰ٷֱȵĹ�����������Ca(OH)2��NaOH�����ź����գ��ɾ��ҷų�H2�� |
| ��ʵ�� | 1g��0.036mo1��Si��20mL����lgNaOH��0.025mol������Һ��С�ļ��ȣ���Ԥ�ȣ����ռ���Լ1700mL H2���ܽӽ�����ֵ��1600mL���� |
��.�ڹ�ҵ������þ��ȡ�裺2Mg��SiO2
 2MgO��Si��ͬʱ�и���Ӧ������2Mg��Si
2MgO��Si��ͬʱ�и���Ӧ������2Mg��Si Mg2Si��Mg2Si������Ѹ�ٷ�Ӧ����SiH4(����)��SiH4�ڳ�������һ�ֲ��ȶ����ֽ�����塣��ͼ�ǽ���Mg��SiO2��Ӧ��ʵ��װ�ã�
Mg2Si��Mg2Si������Ѹ�ٷ�Ӧ����SiH4(����)��SiH4�ڳ�������һ�ֲ��ȶ����ֽ�����塣��ͼ�ǽ���Mg��SiO2��Ӧ��ʵ��װ�ã�
���������Ĵ��ڶԸ�ʵ���нϴ�Ӱ�죬ʵ����Ӧͨ������X��Ϊ���������Թ��еĹ���ҩƷ��ѡ��________(�����)�� a��ʯ��ʯ������b��п��������c������
��4��ʵ�鿪ʼʱ��������ͨ��X���壬�ټ��ȷ�Ӧ���������______________________________������Ӧ��ʼ�����߾ƾ��Ʒ�Ӧ�ܼ������У���ԭ����___________________________��
��5����Ӧ��������ȴ������ʱ������Ӧ��Ļ�����м���ϡ���ᡣ�ɹ۲쵽�����Ļ��ǣ������������ԭ���û�ѧ����ʽ��ʾΪ_______________________________________��
��Ϥʵ������������ȷ����ʵ����������û�ѧʵ���ǰ�ᡣ
��1�������й�ʵ�������ʵ����ʵ����������ȷ���� (�����)��
A��ʵ������Ũ����Ӧ��������ɫϸ��ƿ�У���������ͼ��ʾ��ǩ |
| B����50mL��Ͳ��ȡ5��6mLŨ���� |
| C���к͵ζ�ʵ��ʱ����ƿϴ�Ӹɾ����ñ�Һ��ϴ����ע�����Һ |
| D�������Ȼ�̼��ȡ��ˮ�еĵ⣬��Һʱ�л���ӷ�Һ©�����¶˷ų� |
��2����ͼ��ʵ�����Ʊ�������̽�������Ƿ����Ư���Ե�ʵ��װ��(�гּ�����������ʡ��)��
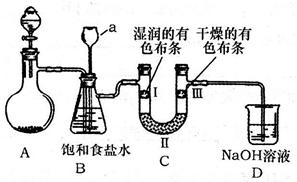
��Aװ���з�Ӧ�Ļ�ѧ����ʽΪ ��
��Bװ��������a�������� ��
��Bװ�õ������dz�ȥ�����л��е�HCl������ȫƿ�����ã�������a��Һ�治
������ʱ��˵�� ����ʱӦֹͣʵ�顣
��ʵ���й۲쵽 ��˵������������Ư���ԡ�


 MnCl2+Cl2��+2H2O������6 mol��HCl�μӷ�Ӧ����ת�Ƶĵ�������Ϊ ��
MnCl2+Cl2��+2H2O������6 mol��HCl�μӷ�Ӧ����ת�Ƶĵ�������Ϊ ��
 ������N2ʱ����Ҫ�İ�������ʯ�Һ�Ũ��ˮ��ԭ����ȡ�����˲������������е�____________����A��B��C������ͬ����NH3�ķ�������Ҫ��ȡ���ռ�N2������ѡ�õ���������___________��
������N2ʱ����Ҫ�İ�������ʯ�Һ�Ũ��ˮ��ԭ����ȡ�����˲������������е�____________����A��B��C������ͬ����NH3�ķ�������Ҫ��ȡ���ռ�N2������ѡ�õ���������___________�� ������ȡN2��������һ�����к����������� ______________________
������ȡN2��������һ�����к����������� ______________________ �������ʹ��Խ��Խ�ܵ����ǵĹ�ע�����ַ�����
�������ʹ��Խ��Խ�ܵ����ǵĹ�ע�����ַ����� ������ȣ�����Խ������____________��
������ȣ�����Խ������____________��