��Ŀ����
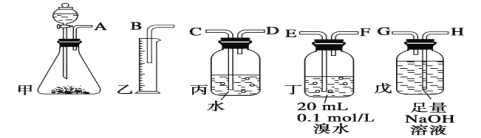
����Ŀ����ˮ�ǿ����ۺ����õġ��Ӻ�ˮ����ȡʳ�κ���Ĺ������£�

��1����д��һ�ֺ�ˮ�����ķ�����__��
��2��������ȡBr2�����ӷ���ʽΪ___��
��3���������SO2ˮ��Һ����Br2��ʹ������ת��Ϊ�������Դﵽ������Ŀ�ġ��䷴Ӧ�Ļ�ѧ����ʽΪBr2��SO2��2H2O=H2SO4��2HBr���ڸ÷�Ӧ�У���������__(�ѧʽ)������Ӧ������2molHBr��������___molSO2��
��4������������Ӧ�ж�SO2��Cl2��Br2����������������ǿ������˳����__��
���𰸡����� Cl2��2Br��=Br2��2Cl�� Br2 1 Cl2>Br2>SO2
��������
��1����ˮ�����ķ����ܶ࣬�����ú�ˮ�еĽ�������������뿪����ͨ�����ӽ������������õ���������Ҳ��������
��2��������ȡBr2�ķ�Ӧ�������������ӷ�����Ӧ��
��3��Br2��SO2��2H2O=H2SO4��2HBr���ڸ÷�Ӧ�У�Br2�е�BrԪ�ؼ�̬���ͣ�SO2�е�SԪ�ؼ�̬���ߣ��ӷ�Ӧʽ��������2molHBr������1molSO2��
��4��SO2��Cl2��Br2�������������Թ�ϵ�������÷�Ӧ���������������Դ�����������������Խ����ж�.
��1����ˮ�����ķ����������Ϊ�����𰸣�����
��2��������ȡBr2�����ӷ���ʽΪCl2��2Br��=Br2��2Cl������Ϊ��Cl2��2Br��=Br2��2Cl����
��3���ڸ÷�ӦBr2��SO2��2H2O=H2SO4��2HBr�У���������Br2������Ӧ������2molHBr��������1molSO2����Ϊ��Br2��1��
��4���ڷ�ӦCl2��2Br��=Br2��2Cl���У�������Cl2>Br2���ڷ�ӦBr2��SO2��2H2O=H2SO4��2HBr�У�������Br2>SO2������SO2��Cl2��Br2����������������ǿ������˳����Cl2>Br2>SO2����Ϊ��Cl2>Br2>SO2��

 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д�����Ŀ��ijʵ��С����0.50mol��L��1NaOH��Һ��0.50mol��L��1��������к��ȵIJⶨ���ⶨϡ�����ϡ����������Һ��Ӧ���к��ȵ�ʵ��װ����ͼ��ʾ��

��1������A������Ϊ___��
��2��װ��������ĭ���ϵ�������___��
��3��д����ʾ�÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ(�к���Ϊ57.3kJ��mol��1)��___��
��4��ȡ50mLNaOH��Һ��30mL�������ʵ�飬ʵ���������±���
ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ� (t2��t1)/�� | ||
1 | H2SO4 | NaOH | ƽ��ֵ | ||
1 | 26.6 | 26.6 | 26.6 | 29.1 | |
2 | 27.0 | 27.4 | 27.2 | 31.2 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 30.4 | |
���¶Ȳ�ƽ��ֵΪ___�档
�ڽ�����Ϊ0.50mol��L��1NaOH��Һ��0.50mol��L��1������ܶȶ���1g��cm��3���кͺ�������Һ�ı�����c��4.18J��(g����)��1�����к��ȡ�H��-53.5kJ/mol��
�����������57.3 kJ��mol��1��ƫ�������ƫ���ԭ�������___(����ĸ)��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c��һ����NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳⶨ������¶�