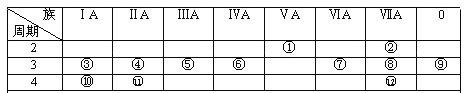
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ Β―ι “¥”Κ§ΒβΖœ“ΚΘ®≥ΐH2OΆβΘ§Κ§”–CCl4ΓΔI2ΓΔI- Β»Θ©÷–ΜΊ ’ΒβΘ§ Β―ιΙΐ≥Χ»γœ¬ΘΚ
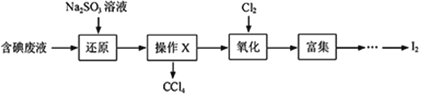
Θ®1Θ©œρΚ§ΒβΖœ“Κ÷–Φ”»κ…‘ΙΐΝΩΒΡNa2SO3»ή“ΚΘ§ΫΪΖœ“Κ÷–ΒΡI2ΜΙ‘≠ΈΣIΘ≠Θ§ΤδάκΉ”ΖΫ≥Χ ΫΈΣ___________ΘΜΗΟ≤ΌΉςΫΪI2ΜΙ‘≠ΈΣIΘ≠ΒΡΡΩΒΡ «________ΓΘ
Θ®2Θ©≤ΌΉςXΒΡΟϊ≥ΤΈΣ__________ΓΘ
Θ®3Θ©―θΜ· ±Θ§‘Ύ»ΐΨ±ΤΩ÷–ΫΪΚ§IΘ≠ΒΡΥ°»ή“Κ”Ο―ΈΥαΒς÷ΝpH‘ΦΈΣ2Θ§ΜΚ¬ΐΆ®»κCl2Θ§‘Ύ40ΓφΉσ”“Ζ¥”ΠΘ® Β―ιΉΑ÷Ο»γΆΦΥυ ΨΘ©ΓΘ Β―ιΩΊ÷Τ‘ΎΫœΒΆΈ¬Ε»œ¬Ϋχ––ΒΡ‘≠“ρ «________ΘΜ“«ΤςaΒΡΟϊ≥ΤΈΣ__________ΘΜ“«Τςb÷– ΔΖ≈ΒΡ»ή“ΚΈΣ________ΓΘ
Θ®4Θ©“―÷ΣΘΚ![]() ΘΜΡ≥Κ§ΒβΖœΥ°Θ®pH‘ΦΈΣ8Θ©÷–“ΜΕ®¥φ‘ΎI2Θ§Ω…Ρή¥φ‘ΎIΘ≠ΓΔ
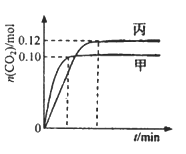
ΘΜΡ≥Κ§ΒβΖœΥ°Θ®pH‘ΦΈΣ8Θ©÷–“ΜΕ®¥φ‘ΎI2Θ§Ω…Ρή¥φ‘ΎIΘ≠ΓΔ![]() ÷–ΒΡ“Μ÷÷ΜρΝΫ÷÷ΓΘ«κ≤Ι≥δΆξ’ϊΦλ―ιΚ§ΒβΖœΥ°÷– «ΖώΚ§”–IΘ≠ΓΔIO3-ΒΡ Β―ιΖΫΑΗΘ® Β―ι÷–Ω…Ι©―Γ‘ώΒΡ ‘ΦΝΘΚœΓ―ΈΥαΓΔΒμΖέ»ή“ΚΓΔFeCl3»ή“ΚΓΔNa2SO3»ή“ΚΘ©ΓΘ
÷–ΒΡ“Μ÷÷ΜρΝΫ÷÷ΓΘ«κ≤Ι≥δΆξ’ϊΦλ―ιΚ§ΒβΖœΥ°÷– «ΖώΚ§”–IΘ≠ΓΔIO3-ΒΡ Β―ιΖΫΑΗΘ® Β―ι÷–Ω…Ι©―Γ‘ώΒΡ ‘ΦΝΘΚœΓ―ΈΥαΓΔΒμΖέ»ή“ΚΓΔFeCl3»ή“ΚΓΔNa2SO3»ή“ΚΘ©ΓΘ
ΔΌ»Γ ΝΩΚ§ΒβΖœΥ°”ΟCCl4Εύ¥ΈίΆ»ΓΓΔΖ÷“ΚΘ§÷±ΒΫΥ°≤ψ”ΟΒμΖέ»ή“ΚΦλ―ι≤Μ≥ω”–ΒβΒΞ÷ ¥φ‘ΎΘΜ
ΔΎ______________________ΘΜ
ΔέΝμ¥”Υ°≤ψ÷–»Γ…ΌΝΩ»ή“ΚΘ§Φ”»κ1-2mLΒμΖέ»ή“ΚΘ§Φ”―ΈΥαΥαΜ·ΚσΘ§ΒΈΦ”Na2SO3»ή“ΚΘ§»τ»ή“Κ±δάΕΥΒΟςΖœΥ°÷–Κ§”–IO3-ΘΜΖώ‘ρΥΒΟςΖœΥ°÷–≤ΜΚ§”–IO3-ΓΘ
Θ®5Θ©Εΰ―θΜ·¬»Θ®ClO2Θ§ΜΤ¬Χ…Ϊ“Ή»ή”ΎΥ°ΒΡΤχΧεΘ© «ΗΏ–ßΓΔΒΆΕΨΒΡœϊΕΨΦΝΚΆΥ°¥ΠάμΦΝΓΘœ÷”Ο―θΜ·Υα–‘Κ§Ζœ“ΚΜΊ ’ΒβΓΘΆξ≥…ClO2―θΜ·IΘ≠ΒΡάκΉ”ΖΫ≥Χ ΫΘΚ_____ClO2+ ____IΘ≠+_______=_____![]() +_____ClΘ≠+Θ®_________Θ©________ΓΘ
+_____ClΘ≠+Θ®_________Θ©________ΓΘ
Θ®6Θ©ΓΑΒβΝΩΖ®Γ± «“Μ÷÷≤βΕ®S2Θ≠Κ§ΝΩΒΡ”––ßΖΫΖ®ΓΘΝΔΒ¬ΖέZnSΓΛBaSO4 «“Μ÷÷≥Θ”ΟΒΡΑΉ…Ϊ―’ΝœΘ§÷Τ±ΗΙΐ≥Χ÷–ΜαΦ”»κΩ…»ή–‘ΒΡBaSΘ§œ÷”ΟΓΑΒβΝΩΖ®Γ±ά¥≤βΕ®ΝΔΒ¬Ζέ―υΤΖ÷–S2Θ≠ΒΡΚ§ΝΩΓΘ≥Τ»Γm g―υΤΖΘ§÷Ο”ΎΒβΝΩΤΩ÷–Θ§“Τ»Γ25.00mL 0.1000mol/L ΒΡI2-KI»ή“Κ”ΎΤδ÷–Θ§≤ΔΦ”»κ““Υα»ή“ΚΘ§Οή±’Θ§÷ΟΑΒ¥ΠΖ¥”Π5minΘ§”–ΒΞ÷ ΝρΈω≥ωΓΘ“‘ΒμΖέΈΣ÷Η ΨΦΝΘ§ΙΐΝΩΒΡI2”Ο0.1000mol/L Na2S2O3 ΒΈΕ®Θ§Ζ¥”Π ΫΈΣ![]() ΓΘ≤βΕ®œϊΚΡNa2S2O3»ή“ΚΧεΜΐV mLΓΘΝΔΒ¬Ζέ―υΤΖS2Θ≠Κ§ΝΩΈΣ__________Θ®–¥≥ω±μ¥ο ΫΘ©
ΓΘ≤βΕ®œϊΚΡNa2S2O3»ή“ΚΧεΜΐV mLΓΘΝΔΒ¬Ζέ―υΤΖS2Θ≠Κ§ΝΩΈΣ__________Θ®–¥≥ω±μ¥ο ΫΘ©
ΓΨ¥πΑΗΓΩ![]() ΙCCl4÷–ΒΡΒβΫχ»κΥ°≤ψ Ζ÷“Κ Ι¬»Τχ‘Ύ»ή“Κ÷–”–Ϋœ¥σΒΡ»ήΫβΕ» «ρ–ΈάδΡΐΙή NaOH»ή“Κ Υ°≤ψ»Γ…ΌΝΩ»ή“ΚΘ§Φ”»κ1-2mLΒμΖέ»ή“ΚΘ§Φ”»κ―ΈΥαΥαΜ·Θ§ΒΈ»κFeCl3»ή“ΚΘ§»τ»ή“Κ±δάΕ…ΪΘ§ΥΒΟςΖœΥ°÷–Κ§”–I-,Ζώ‘ρ≤ΜΚ§I- 2 10 8H+ 5 2 4 H2O
ΙCCl4÷–ΒΡΒβΫχ»κΥ°≤ψ Ζ÷“Κ Ι¬»Τχ‘Ύ»ή“Κ÷–”–Ϋœ¥σΒΡ»ήΫβΕ» «ρ–ΈάδΡΐΙή NaOH»ή“Κ Υ°≤ψ»Γ…ΌΝΩ»ή“ΚΘ§Φ”»κ1-2mLΒμΖέ»ή“ΚΘ§Φ”»κ―ΈΥαΥαΜ·Θ§ΒΈ»κFeCl3»ή“ΚΘ§»τ»ή“Κ±δάΕ…ΪΘ§ΥΒΟςΖœΥ°÷–Κ§”–I-,Ζώ‘ρ≤ΜΚ§I- 2 10 8H+ 5 2 4 H2O 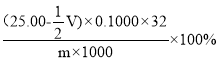
ΓΨΫβΈωΓΩ
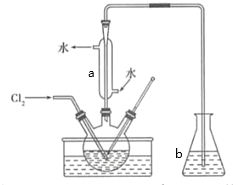
Θ®1Θ©ΒβΨΏ”–―θΜ·–‘Θ§Ρή―θΜ·―«ΝρΥαΡΤ…ζ≥…ΝρΥαΡΤΘ§Ή‘…μ±ΜΜΙ‘≠ΈΣΒβάκΉ”Θ§άκΉ”Ζ¥”ΠΖΫ≥Χ ΫΈΣSO32Θ≠+ I2 + H2O = 2IΘ≠+ SO42Θ≠+ 2H+ΘΜΒβΈΔ»ή”ΎΥ°Θ§ΕχΒβάκΉ”“Ή»ή”ΎΥ°Θ§ΈΣΝΥ ΙΗϋΕύΒΡΒβ‘ΣΥΊΫχ»κΥ°»ή“Κ÷–”ΠΗΟΫΪΒβΜΙ‘≠ΈΣΒβάκΉ”Θ§ ΙCCl4÷–ΒΡΒβΫχ»κΥ°≤ψΘΜ
Θ®2Θ©ΥΡ¬»Μ·ΧΦ τ”Ύ”–ΜζΈοΘ§Υ° τ”ΎΈόΜζΈοΘ§Εΰ’Ώ≤ΜΜΞ»ήΘ§Ζ÷άκΜΞ≤Μœύ»ήΒΡ“ΚΧεΘ§”ΟΖ÷“ΚΒΡΖΫΖ®ΘΜ
Θ®3Θ©Ββ»ί“Ή…ΐΜΣΘ§«“¬»ΤχΒΡ»ήΫβΕ»ΥφΉ≈Έ¬Ε»ΒΡ…ΐΗΏΕχΦθ–ΓΘ§Έ¬Ε»‘ΫΗΏΘ§¬»ΤχΒΡ»ήΫβΕ»‘Ϋ–ΓΘ§Ζ¥”Π‘Ϋ≤Μ≥δΖ÷Θ§Υυ“‘”ΠΗΟ‘ΎΒΆΈ¬ΧθΦΰœ¬Ϋχ––Ζ¥”ΠΘ§¬»ΤχΚΆΒβ’τΤχΕΦ”–ΕΨΘ§≤ΜΡή÷±Ϋ”≈≈»κΩ’ΤχΘ§«“ΕΦΡή”κ«β―θΜ·ΡΤ»ή“ΚΖ¥”Π…ζ≥…ΈόΕΨΈο÷ ΘΜ“«ΤςaΒΡΟϊ≥ΤΈΣ«ρ–ΈάδΡΐΙήΘΜ“«Τςb÷– ΔΖ≈ΒΡ»ή“ΚΈΣNaOH»ή“ΚΘ§Έϋ ’Ρ©Ζ¥”ΠΒΡ¬»ΤχΘΜ
Θ®4Θ©ΒβάκΉ”ΨΏ”–ΜΙ‘≠–‘Θ§Ρή±Μ―θΜ·ΦΝ―θΜ·…ζ≥…ΒβΘ§ΒβΥαΗυάκΉ”ΨΏ”–―θΜ·–‘Θ§Ρή±ΜΜΙ‘≠ΦΝΜΙ‘≠…ζ≥…ΒβΘ§Ββ”ωΒμΖέ ‘“Κ±δάΕ…ΪΘ§Υυ“‘ΤδΦλ―ιΖΫΖ®ΈΣΘΚ¥”Υ°≤ψ»Γ…ΌΝΩ»ή“ΚΘ§Φ”»κ1-2mLΒμΖέ»ή“ΚΘ§Φ”»κ―ΈΥαΥαΜ·Θ§ΒΈΦ”FeCl3»ή“ΚΘ§2I-+2Fe3+=2Fe2++I2Θ§»τ»ή“Κ±δάΕ…ΪΘ§ΥΒΟςΖœΥ°÷–Κ§”–I-Θ§Ζώ‘ρ≤ΜΚ§I-ΘΜΝμ¥”Υ°≤ψ»Γ…ΌΝΩ»ή“ΚΘ§Φ”»κ1-2mLΒμΖέ ‘“ΚΘ§Φ”―ΈΥαΥαΜ·Θ§ΒΈΦ”Na2SO3»ή“ΚΘ§5SO32-+2 IO3-+2H+=I2+5SO42-+H2OΘ§»τ»ή“Κ±δάΕ…ΪΘ§ΥΒΟςΖœΥ°÷–Κ§”–IO3-Θ§Ζώ‘ρ≤ΜΚ§IO3-ΘΜ
Θ®5Θ©ΔΌ”ΟClO2―θΜ·Υα–‘Κ§I-Ζœ“ΚΜΊ ’ΒβΘ§ «Εΰ―θΜ·¬»‘ΎΥα»ή“Κ÷–―θΜ·ΒβάκΉ”…ζ≥…ΒβΒΞ÷ Θ§Εΰ―θΜ·¬»±ΜΜΙ‘≠ΈΣ¬»άκΉ”Θ§ClO2ΓΪCl-ΓΪ5e-Θ§2I-ΓΪI2ΓΪ2e-Θ§Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣΘΚ2ClO2+10I-+8H+=5I2+2Cl-+4H2OΘΜ
Θ®6Θ©ΗυΨίΜ·ΚœΦέ…ΐΫΒœύΒ»Ν–ΙΊœΒ ΫΘ§…ηΝράκΉ”Έο÷ ΒΡΝΩΈΣn molΘ§‘ρ
S2-~~~~~~~~~I2 ΓΓΓΓΓΓΓΓΓΓ 2S2O32-~~~~~~~~~~~ I2
1ΓΓΓΓ 1ΓΓΓΓΓΓ ΓΓ2 ΓΓΓΓ 1
n mol n mol 0.1VΓΝ10-3mol ![]() ΓΝ0.1VΓΝ10-3mol
ΓΝ0.1VΓΝ10-3mol
n+![]() ΓΝ0.1VΓΝ10-3mol=25ΓΝ0.1VΓΝ10-3molΘ§ΒΟn=(25-
ΓΝ0.1VΓΝ10-3mol=25ΓΝ0.1VΓΝ10-3molΘ§ΒΟn=(25-![]() V)ΓΝ0.1VΓΝ10-3molΘΜ
V)ΓΝ0.1VΓΝ10-3molΘΜ
‘ρ―υΤΖ÷–ΝράκΉ”Κ§ΝΩΈΣΘΚ![]() =
=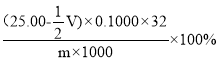 ΓΘ
ΓΘ

 Οϊ–ΘΩΈΧΟœΒΝ–¥πΑΗ
Οϊ–ΘΩΈΧΟœΒΝ–¥πΑΗΓΨΧβΡΩΓΩ”≈Μ·Ζ¥”ΠΧθΦΰ «―–ΨΩΜ·―ßΖ¥”ΠΒΡ÷Ί“ΣΖΫœρΓΘ
Θ®1Θ©“‘Νρ¥ζΝρΥαΡΤ”κΝρΥαΒΡΖ¥”ΠNa2S2O3+H2SO4=Na2SO4+SO2+SΓΐ+H2OΈΣάΐΘ§ΧΫΨΩΆβΫγΧθΦΰΕ‘Μ·―ßΖ¥”ΠΥΌ¬ ΒΡ”ΑœλΘ§ Β―ιΖΫΑΗ»γœ¬±μΥυ ΨΓΘ
Β―ι–ρ Κ≈ | Na2S2O3»ή“Κ | H2SO4»ή“Κ | ’τΝσΥ° | Έ¬Ε»/Γφ | ||
≈®Ε»/Θ®mol/LΘ© | ΧεΜΐ/mL | ≈®Ε»/Θ®mol/LΘ© | ΧεΜΐ/mL | ΧεΜΐ/mL | ||
Δώ | 0.1 | 1.5 | 0.1 | 1.5 | 10 | 20 |
Δρ | 0.1 | 2.5 | 0.1 | 1.5 | 9 | a |
Δσ | 0.1 | b | 0.1 | 1.5 | 9 | 30 |
ΔΌ±μ÷–Θ§aΈΣ______Θ§bΈΣ______ΓΘ
ΔΎ Β―ι±μΟςΘ§ Β―ιΔσΒΡΖ¥”ΠΥΌ¬ ΉνΩλΘ§÷ß≥÷’β“ΜΫα¬έΒΡ Β―ιœ÷œσΈΣ_______ΓΘ
ΔέΝρ¥ζΝρΥαΡΤΩ…”Ο”Ύ¥”Κ§―θΜ·“χΒΡΩσ‘ϋ÷–Ϋΰ≥ω“χΘ§Ζ¥”Π»γœ¬ΘΚAg2O+4S2O32-+H2O2[Ag(S2O3)2]3-+2OH-ΓΘ‘Ύ ΒΦ …ζ≤ζ÷–Θ§ΈΣΝΥΧαΗΏ“χΒΡΫΰ≥ω¬ –η“ΣΒςΫΎpHΒΡΖΕΈßΈΣ8.5~9.5Θ§Ϋβ ΆΤδ‘≠“ρΘΚ_________ΓΘ
Θ®2Θ©ΙΛ“Β…œ≥Θ”ΟΩ’Τχ¥ΏΜ·―θΜ·Ζ®≥ΐ»ΞΒγ ·‘ϋΫ§Θ®Κ§ CaOΘ©…œ«ε“Κ÷–ΒΡS2-Θ§≤Δ÷Τ»Γ ·ΗύΘ®CaSO42H2OΘ©Θ§Τδ÷–ΒΡΈο÷ ΉΣΜ·Ιΐ≥Χ»γΆΦΥυ ΨΓΘ

ΔΌΙΐ≥ΧΔώΓΔΔρ÷–Θ§Τπ¥ΏΜ·ΦΝΉς”ΟΒΡΈο÷ «_________ΓΘ
ΔΎΙΐ≥ΧΔρ÷–Θ§Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ________ΓΘ
ΔέΗυΨίΈο÷ ΉΣΜ·Ιΐ≥ΧΘ§»τΫΪ10L…œ«ε“Κ÷–ΒΡS2-ΉΣΜ·ΈΣSO42-Θ®S2-≈®Ε»ΈΣ320mg/LΘ©Θ§άμ¬έ…œΙ≤–η“Σ±ξΉΦΉ¥Ωωœ¬ΒΡO2ΒΡΧεΜΐΈΣ_______LΓΘ
ΓΨΧβΡΩΓΩ“ΜΕ®Έ¬Ε»œ¬Θ§‘Ύ»ΐΗω»ίΜΐΨυΈΣ2.0 LΒΡΚψ»ίΟή±’»ίΤς÷–ΖΔ…ζΖ¥”ΠΘΚ2NO(g)+2CO(g) ![]() N2(g)+2CO2(g),Ης»ίΤς÷–Τπ ΦΈο÷ ΒΡΝΩ≈®Ε»”κΖ¥”ΠΈ¬Ε»»γœ¬±μΥυ ΨΘ§Ζ¥”ΠΙΐ≥Χ÷–ΦΉΓΔ±ϊ»ίΤς÷–CO2ΒΡΈο÷ ΒΡΝΩΥφΦδ±δΜ·ΙΊœΒ»γœ¬ΆΦΥυ ΨΓΘ
N2(g)+2CO2(g),Ης»ίΤς÷–Τπ ΦΈο÷ ΒΡΝΩ≈®Ε»”κΖ¥”ΠΈ¬Ε»»γœ¬±μΥυ ΨΘ§Ζ¥”ΠΙΐ≥Χ÷–ΦΉΓΔ±ϊ»ίΤς÷–CO2ΒΡΈο÷ ΒΡΝΩΥφΦδ±δΜ·ΙΊœΒ»γœ¬ΆΦΥυ ΨΓΘ
»ίΤς | Έ¬Ε»/Γφ | Τπ ΦΈο÷ ΒΡΝΩ≈®Ε»/molΓΛL-1 | |||
NO(g) | CO(g) | N2 | CO2 | ||
ΦΉ | T1 | 0.10 | 0.10 | 0 | 0 |
““ | T1 | 0 | 0 | 0.10 | 0.20 |
±ϊ | T2 | 0.10 | 0.10 | 0 | 0 |
œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
A. ΗΟΖ¥”ΠΒΡ’ΐΖ¥”ΠΈΣΈϋ»»Ζ¥”Π
B. ““»ίΤς÷–Ζ¥”Π¥οΒΫΤΫΚβ ±Θ§N2ΒΡΉΣΜ·¬ ¥σ”Ύ40%
C. ¥οΒΫΤΫΚβ ±Θ§““»ίΤς÷–ΒΡ―Ι«Ω“ΜΕ®¥σ”ΎΦΉ»ίΤςΒΡ2±Ε
D. ±ϊ»ίΤς÷–Ζ¥”Π¥οΒΫΤΫΚβΚσΘ§‘Ό≥δ»κ0.10mol NOΚΆ0.10mol CO2Θ§¥Υ ±v(’ΐ)ΘΨv(Ρφ)
ΓΨΧβΡΩΓΩΡ≥ΩΈΆβ–Υ»Λ–ΓΉιΫχ––ΒγΫβ‘≠άμΒΡ Β―ιΧΫΨΩΘ§ΉωΝΥ»γœ¬ΒΡ Β―ιΘΚ“‘Ά≠ΈΣΒγΦΪΘ§Α¥»γΆΦΥυ ΨΒΡΉΑ÷ΟΒγΫβ±ΞΚΆ ≥―ΈΥ°ΓΘ

Β―ιœ÷œσΘΚΫ”Ά®Βγ‘¥30 sΡΎΘ§―τΦΪΗΫΫϋ≥ωœ÷ΑΉ…ΪΜκΉ«Θ§÷°Κσ±δ≥…≥»ΜΤ…ΪΜκΉ«Θ§¥Υ ±≤βΕ®»ή“ΚΒΡpH‘ΦΈΣ10ΓΘ“ΜΕΈ ±ΦδΚσΘ§ ‘ΙήΒΉ≤ΩΨέΦ·¥σΝΩΚλ…Ϊ≥ΝΒμΘ§»ή“Κ»‘ΈΣΈό…ΪΓΘ
≤ι‘ΡΉ ΝœΘΚ
Έο÷ | ¬»Μ·Ά≠ | ―θΜ·―«Ά≠ | «β―θΜ·―«Ά≠(≤ΜΈ»Ε®) | ¬»Μ·―«Ά≠ |
―’…Ϊ | ΙΧΧε≥ ΉΊ…ΪΘ§≈®»ή“Κ≥ ¬Χ…ΪΘ§œΓ»ή“Κ≥ άΕ…Ϊ | Κλ…Ϊ | ≥»ΜΤ…Ϊ | ΑΉ…Ϊ |
*œύΆ§Έ¬Ε»œ¬CuClΒΡ»ήΫβΕ»¥σ”ΎCuOH
œ¬Ν–ΥΒΖ®¥μΈσΒΡ «
A. Ζ¥”ΠΫα χΚσΉν÷’»ή“Κ≥ Φν–‘
B. “θΦΪ…œΖΔ…ζΒΡΒγΦΪΖ¥”ΠΈΣΘΚ2H2O + 2e®T H2Γϋ+ 2OH
C. ΒγΫβΙΐ≥Χ÷–¬»άκΉ”“Τœρ―τΦΪ
D. ‘ΙήΒΉ≤ΩΚλ…ΪΒΡΙΧΧεΨΏ”–ΜΙ‘≠–‘
ΓΨΧβΡΩΓΩΕΰ―θΜ·¬»Θ®ClO2Θ© «“Μ÷÷–¬–ΆœϊΕΨΦΝΘ§Ω…”Ο¬»ΥαΡΤΘ®NaClO3Θ©ΈΣ‘≠Νœ÷Τ±ΗΓΘ
Θ®1Θ©ΗτΡΛΒγΫβΖ®÷Τ±ΗClO2ΒΡΉΑ÷Ο Ψ“βΆΦ»γœ¬ΘΚ

“―÷ΣΘΚClO2‘ΎΥα–‘»ή“Κ÷–±»ΫœΈ»Ε®Θ§‘ΎΦν–‘»ή“Κ÷–≤ΜΡήΈ»Ε®¥φ‘ΎΓΘ
ΔΌ≤ζ…ζO2ΒΡΒγΦΪΖ¥”Π ΫΘΚ________ΓΘ
ΔΎΫαΚœΖ¥”ΠΖΫ≥Χ ΫΘ§Φρ ωClO2ΒΡ≤ζ…ζΙΐ≥ΧΘΚ_________ΓΘ
Θ®2Θ©Ιΐ―θΜ·«βΜΙ‘≠Ζ®÷Τ±ΗClO2ΘΚ![]() ―–ΨΩΖΔœ÷Cl-Ε‘…œ ωΖ¥”Π”–”ΑœλΘ§ Β―ιΦ«¬Φ»γœ¬ΘΚ
―–ΨΩΖΔœ÷Cl-Ε‘…œ ωΖ¥”Π”–”ΑœλΘ§ Β―ιΦ«¬Φ»γœ¬ΘΚ
Φ”»κNaCl ΒΡ≈®Ε»/(gΓΛL1) | ClO2ΒΡ…ζ≥…ΥΌ¬ /(gΓΛL-1ΓΛmin-1) | œύΆ§ ±Φδ | |||
10 min | 30 min | 60 min | ClO2 ≤ζ¬ /% | Cl2 ΒΡΝΩ | |
0 | 0.0035 | 0.0124 | 0.0159 | 97.12 | ΦΪΈΔΝΩ |
1.00 | 0.0138 | 0.0162 | 0.0163 | 98.79 | ΦΪΈΔΝΩ |
ΔΌNaClΒΡ÷ς“ΣΉς”Ο «_______ΓΘ
ΔΎ…œ ωΖ¥”ΠΩ…ΡήΒΡΙΐ≥Χ»γœ¬ΘΚ
Ζ¥”ΠiΘΚ![]() +
+ ![]() + +
+ +
Ζ¥”ΠiiΘΚ Cl2 + H2O2 = 2Cl- + O2Γϋ + 2H+
ΫΪΖ¥”ΠiΧν–¥Άξ’ϊ_________ΓΘ
ΔέΫχ“Μ≤Ϋ―–ΨΩΖΔœ÷Θ§Έ¥ΧμΦ”Cl- ±Θ§ΧεœΒ÷– Ήœ»ΜαΖΔ…ζΖ¥”Π…ζ≥…Cl-Θ§Ζ¥”ΠΈΣΘΚClO3- + 3H2O2 = Cl- + 3O2Γϋ+3H2OΘ®Ζ¥”ΠiiiΘ©ΓΘΖ÷ΈωΖ¥”ΠiΓΔiiΓΔiiiΒΡΥΌ¬ ¥σ–ΓΙΊœΒ≤ΔΦρ“ΣΥΒΟςάμ”…ΘΚ_________ΓΘ
Θ®3Θ©ΙζΦ“ΙφΕ®Θ§“ϊ”ΟΥ°÷–ClO2ΒΡ≤–ΝτΝΩ≤ΜΒΟΗΏ”Ύ0.8 mgΓΛL-1Θ§Φλ≤β≤Ϋ÷η»γœ¬ΘΚ
ΔώΘ°»Γ1.0LΒΡΥα–‘Υ°―υΘ§Φ”»κΙΐΝΩΒΡΒβΜ·ΦΊΘ§‘Ό”Ο«β―θΜ·ΡΤ»ή“ΚΒς÷Ν÷––‘Θ§ ΙClO2ΉΣΜ·ΈΣ![]() ΓΘΦ”»κΒμΖέ»ή“ΚΘ§»ή“Κ±δάΕΓΘ
ΓΘΦ”»κΒμΖέ»ή“ΚΘ§»ή“Κ±δάΕΓΘ
ΔρΘ°”Ο0.0010 mol/LNa2S2O3»ή“ΚΒΈΕ®≤Ϋ÷ηI÷–≤ζ…ζΒΡI2ΓΘ“―÷ΣΘΚ≤Ϋ÷ηII÷–ΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «![]()
ΔΌ≤Ϋ÷ηI÷–ΖΔ…ζΒΡ―θΜ·ΜΙ‘≠Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «________ΓΘ
ΔΎΒ±≤Ϋ÷ηII÷–≥ωœ÷________Θ®Χνœ÷œσΘ© ±Θ§ΆΘ÷ΙΒΈΦ”Na2S2O3»ή“ΚΘ§Φ«¬ΦΤδΧεΜΐΈΣ10.00 mLΓΘ
Δέ…œ ωΥ°―υ÷–ClO2ΒΡ≤–Ντ≈®Ε» «______mgΓΛL-1ΓΘ