��Ŀ����
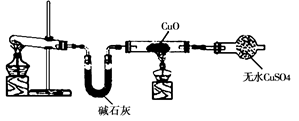
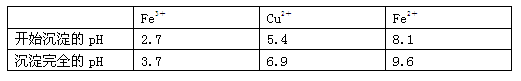
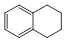
1,2,3,4�����⻯���Ľṹ��ʽ����ͼ, ����ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+ 4Br2�� C10H8Br4+ 4HBr���ɵ����廯��������Ϊ��̬��������ˮ�����������⻯����Һ�塢����ˮ�ʹ�����Ϊԭ�ϣ��Ʊ�����������������Һ��ʵ�鲽�����£�
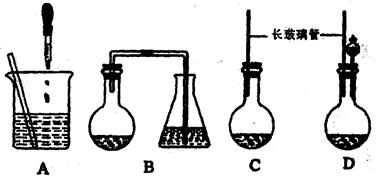
�ٰ�һ�������Ȱ����⻯����ˮ�����ʵ��������У��������������ۡ�
����������Һ�壬���Ͻ��裬ֱ����Ӧ��ȫ��
��ȡ�·�Ӧ�����������������⻯����ֱ����Һ��ɫ��ʧ�����ˣ�����Һ�����Һ©�������á�
�� ��Һ���õ��ġ�ˮ�㡱����������Һ��
�ش���������:
(1) ��ͼ��ʾ��װ��, �ʺϲ���ٺ͢ڵIJ������� ��
(2) �����������жϡ���Ӧ��ȫ��_________________________________________��
(3) ������в����������⻯����Ŀ���� ��
(4) ������й��˺�õ��Ĺ���������____________ _________��
(5) ��֪��ʵ�������£�������������Һ�������������������66%������廯��Ӧ������ȫ���������ˮ�����⻯����������Լ��1:____________(����С�����1λ)��


�ٰ�һ�������Ȱ����⻯����ˮ�����ʵ��������У��������������ۡ�
����������Һ�壬���Ͻ��裬ֱ����Ӧ��ȫ��
��ȡ�·�Ӧ�����������������⻯����ֱ����Һ��ɫ��ʧ�����ˣ�����Һ�����Һ©�������á�
�� ��Һ���õ��ġ�ˮ�㡱����������Һ��
�ش���������:
(1) ��ͼ��ʾ��װ��, �ʺϲ���ٺ͢ڵIJ������� ��
(2) �����������жϡ���Ӧ��ȫ��_________________________________________��
(3) ������в����������⻯����Ŀ���� ��
(4) ������й��˺�õ��Ĺ���������____________ _________��
(5) ��֪��ʵ�������£�������������Һ�������������������66%������廯��Ӧ������ȫ���������ˮ�����⻯����������Լ��1:____________(����С�����1λ)��
�� A �� ��Һ����ɫ����ɫ(�Ⱥ�) �dz�ȥ������嵥��
�� ���廯�� �� 0.8
�� ���廯�� �� 0.8
���������(1) ���������IJ���Ҫ���֪���ʺϲ���ٺ͢ڵIJ�������Aѡ�
(2) ����Һ������Һ��������ɫ�����Բ�������жϡ���Ӧ��ȫ���ķ�������Һ����ɫ����ɫ(�Ⱥ�)��
(3)���ڷ�Ӧ���嵥���ǹ����ģ����Բ�����в����������⻯����Ŀ���dz�ȥ������嵥�ʡ�
��4�����ɵ����廯��������Ϊ��̬��������ˮ�����Բ�����й��˺�õ��Ĺ������������廯����
��5��������������Һ�������������������66%���������Һ��������100g������Һ��HBr��������66g��ˮ��������34g�����ݷ�Ӧ�Ļ�ѧ����ʽC10H12+ 4Br2
 C10H8Br4+ 4HBr��֪������66gHBr��Ҫ���⻯����������
C10H8Br4+ 4HBr��֪������66gHBr��Ҫ���⻯����������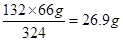 �����Բ������ˮ�����⻯����������Լ��1:0.8��
�����Բ������ˮ�����⻯����������Լ��1:0.8�������������ۺ���ǿ���ѶȽϴ�ѧ�����ۺ���������˸��ߵ�Ҫ������������ѧ���淶��ʵ����ơ��������������Ʊ���ʵ������⣬�ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ��еĻ��ṩһЩ�µ���Ϣ�����Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ�

��ϰ��ϵ�д�
 ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�
�����Ŀ