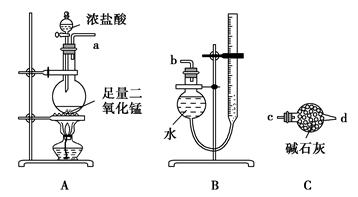
��Ŀ����
(15��)ij�о�С���������װ�÷���CO��CO2�Ļ�����岢̽��CO��ԭ����ͭ�IJ���������Ƶ����ʡ���֪�Ȼ��٣�PdCl2����Һ��CO����������ɫ����(��������)���г�װ��δ������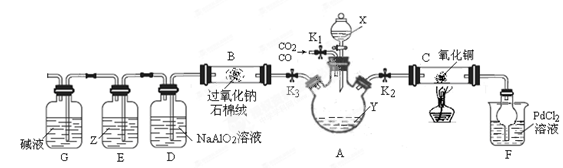
����9ͼ��
��1���Լ�X��Y����ɫ��Ӧ���ʻ�ɫ����X��Y������ �� .
��2������װ�á����������Բ�װ��ҩƷ�رշ�Һ©��������K3����K1��K2�������CO�����۲쵽 ����ʱ���ɶ�Cװ�ý��м��Ȳ�������Ӧ����Ϊ��ɫ���塣
��3����ѯ���ϻ��������Ϣ����Cu2OΪ��ɫ���壻�ڳ����£�Cu2+����Һ���ȶ���Cu+�������������·�����Ӧ��2Cu+ =Cu2++Cu��
�ס�������ͬѧȡ������ɫ����(��M����)����ʵ�飬̽����ɷ֣�
| ��� | ʵ����� | ʵ����������� | �� �� |
| �� | �� ��a g M�м���һ����ϡ���ᣬ��ֽ��裻 �� �����μ�ϡ����������, ��ַ�Ӧ. | �ٹ������Լ��٣� ����Ȼ��һ�������壬��Һ����ɫ | ��M��һ����Cu2O; ��M��һ����Cu. |
| �� | ����ʵ���������Һ���� ������ϴ�ӡ�������� | ��������Ϊ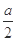 g g | MΪCu��Cu2O�Ļ���� |
(ii)����ͬѧ�������ͬѧ�����˼���,��Ϊ����ͬѧ�Ľ�������ȷ��,���ó�M��Cu��Cu2O�����ʵ���֮���� .
��4��Cװ�÷�Ӧ���������ӻ�������з����CO2������е���Ҫ������ .ʵ�������Dװ�ò�����ɫ���������ӷ���ʽΪ .
��5��Z�Լ�Ϊ����KI�ı���Һ����Eװ���п��ܲ����������� .
��1��NaHSO4 NaOH����1�֣���2�֣���
��2��Fװ���г���������ɫ������2�֣���
��3��M����ֻ��Cu2O,��Cu2O�����������·�Ӧ������Cu��2�֣���1��4 .(3��)
��4���ر�K1��K2����K3�ͷ�Һ©��������2�֣���
2AlO2- + CO2 + 3H2O=2Al(OH)3��+CO32-
��AlO2- + CO2 + 2H2O=Al(OH)3��+HCO3-��2�֣�
��5����Һ�ֲ�,�ϲ�����Ϻ�ɫ����2�֣�
��������������������װ��ͼ֪����K1��CO��CO2�Ļ������ͨ��A���رշ�Һ©��������K3��Y�����ն�����̼��Ӧ����K1��K2��CO������ͭ����������ԭ��Ӧ������֤CO�Ļ�ԭ�ԣ��Ȼ��٣�PdCl2����Һ��CO����������ɫ��������F�г��ֳ�����˵��CO����װ�ã�Ȼ��ɼ��ȣ�Cװ�÷�Ӧ�����ر�K1��K2����K3�ͷ�Һ©����������X����̼���η�Ӧ�������������̼��D�����ɳ���Ϊ������������B�з���������̼��������Ƶķ�Ӧ��������������Z�е�KI�ɱ��������ɵⵥ�ʣ��ܽ��ڱ���Ϊ�Ϻ�ɫ��G�еļ�Һ�ɷ�ֹ�����Ķ�����̼����װ�á���1���Լ�X��Y����ɫ��Ӧ���ʻ�ɫ������NaԪ�أ�X�����ԣ�Y�Լ��ԣ���XΪNaHSO4��YΪNaOH����2��CO����ʱ���ȷ�����ը����Fװ���г���������ɫ������CO����װ�ÿɼ���Cװ�ã���3����i����ʵ����M����ֻ��Cu2O����Cu2O�����������·���������ԭ��Ӧ������Cu��ͬʱ��������ͭ��Һ����ʵ����۲��ɿ�����ii��agM���壬�����ᷴӦ����������Ϊa/2g����Cu��Cu2O�ֱ�Ϊxmol��ymol��Cu+�������������·�����Ӧ��2Cu+=Cu2++Cu����64x+160y��a��64x+64y��a/2�����x��y=1��2����4��������������֪�����ӻ�������з����CO2������е���Ҫ�����ǹر�K1��K2����K3�ͷ�Һ©��������Dװ�ò�����ɫ���������ӷ���ʽΪ2AlO2-+CO2+3H2O=2Al��OH��3��+CO32-��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-����5��Z�е�KI�ɱ��������ɵⵥ�ʣ��ܽ��ڱ���Ϊ�Ϻ�ɫ����Eװ���п��ܲ�������������Һ�ֲ㣬�ϲ�����Ϻ�ɫ��
���㣺������������ʵ�����ơ����������ۡ�

����ʵ�鷽�����ܴﵽʵ��Ŀ�ĵ���
| | ʵ��Ŀ�� | ʵ�鷽�� |
| A | �о������Թ�������ֽ����ʵ�Ӱ�� | �ֱ�����֧�Թ��м�����������Ũ�ȵĹ���������Һ����������һֻ�Թ��м�������MnO2 |
| B | ֤��Mg(OH)2��������ת��ΪFe(OH)3���� | ��2mol/LNaOH��Һ���ȼ���3��1mol/L MgCl2��Һ���ټ���3��1mol/L FeCl3��Һ |
| C | ���Լ�����������Һ | ��Na2CO3��Һ��HCl��Һ��μ� |
| D | �ⶨ�������������ĺ��� | ȡa g����������ϡ�����ַ�Ӧ�����ݳ�������ͨ����ʯ�Һ������ΪbL����״���£� |
ʵ������Ҫ������NaCl��Һ����ʵ���ҵ�NaCl�����������Na2SO4��NH4HCO3��ijͬѧ����������ͼ���ʵ���ȥ���ʣ��ش��������⣺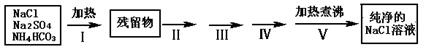
��1������I��ȥ�������ǣ��ѧʽ��_______________��ֱ�Ӽ���Ҫ���ڼ�ǿ����ٽ��м��ȣ������� ��
��2��������ͼ���ʵ����ƣ�����ص�ʵ�������ʵ�������ʵ��Ŀ����д���±��У�
| �������� | ʵ������ | ʵ��Ŀ�� |
| ����II�����������ܽ�õ���Һ�� | | |
| ����III�� |  | |
| ����IV�����ˣ�����Һ�� | | |
| ����V������Һ������� |  | |
��3�������õ�20���NaCl������Һ����֪20��ʱNaCl���ܽ��Ϊ36.0g��NaCl������Һ���ܶ�Ϊ1.12g/cm3 ����20���NaCl������Һ�����ʵ���Ũ��Ϊ mol/L��������������λ��Ч���֣���

 ��ʾ����װ�ý���ʵ�顣ʵ��������£���ͼ��װ���������ڹ��ƿ��ʢ��������H2O2ˮ��Һ���ù��Ϊ20mL����Ͳ����100�Σ�ʹ�����е�SO2��H2O2ˮ��Һ������գ�SO2+H2O2��H2SO4���������պ��ˮ��Һ�м���������BaCl2��Һ�����ɰ�ɫ�����������ˡ�ϴ�ӡ�����Ȳ������г������ð�ɫ����0.182mg��
��ʾ����װ�ý���ʵ�顣ʵ��������£���ͼ��װ���������ڹ��ƿ��ʢ��������H2O2ˮ��Һ���ù��Ϊ20mL����Ͳ����100�Σ�ʹ�����е�SO2��H2O2ˮ��Һ������գ�SO2+H2O2��H2SO4���������պ��ˮ��Һ�м���������BaCl2��Һ�����ɰ�ɫ�����������ˡ�ϴ�ӡ�����Ȳ������г������ð�ɫ����0.182mg��

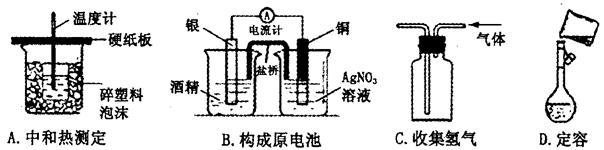
 2CuCl2��O2�ⶨͭ�Ľ������ԭ���������ɹ�ѡ���װ����ͼ��ʾ��
2CuCl2��O2�ⶨͭ�Ľ������ԭ���������ɹ�ѡ���װ����ͼ��ʾ��