��Ŀ����
��16�֣�������ȫ������������ȫ����Ҫ���ϡ�������������ײ��˲�䣬��ȫװ��ͨ����ʹ���еķ�ĩ�ֽ��ͷų������ĵ����γ����ң��Ӷ�����˾�����˿������˺���Ϊ�о���ȫ���ҹ����Ļ�ѧԭ����ȡ��ȫװ���еķ�ĩ����ʵ�顣����ɷ�����ȷ���÷�ĩ��Na��Fe��N��O����Ԫ�ء�ˮ������������������ĩ�����ܽ⡣����⣬������Ϊ������ף�������Ϊ����ɫ���壬���������ᡣ
ȡ13.0g������ף�����ʹ����ȫ�ֽ⣬���ɵ����͵����ң����ɵĵ����ۺϳɱ�״���µ����Ϊ6.72L���������ڸ��¸����������������벻�������ɫ��ĩ��Ӧ���ɵ���ɫ�����������һ�ֵ��ʡ��������������Ӵ���ת��Ϊ�������Ρ�
��ش��������⣺
��1���Ļ�ѧʽΪ �����ĵ���ʽΪ ��
��2�������������ɫ��ĩ������Ӧ�Ļ�ѧ����ʽΪ ��
��3�������ڿ�����ת��Ϊ̼�����Σ���Ӧ�Ļ�ѧ����ʽΪ ��
��4�����������У��п�����Ϊ��ȫ�����к���ɫ��ĩ���Ʒ���� ��
A�� KCl B�� KNO3 C�� Na2S D�� CuO
��5��ijͬѧ��������ڿ�����Ҳ����ת��Ϊ��һ���Ρ������ڿ����з���һ��ʱ���Ϊ̽����ת������ijɷ֣���������·�����
A��ȡa�˻����������ϡ�����ַ�Ӧ���ݳ������ü�ʯ�����գ�����b��
B��ȡa�˻����������ϡ�����ַ�Ӧ�����ȡ����ɡ����գ���b�˹���
C��ȡa�˻�����ּ��ȣ���b�˹���
D��ȡa�˻����������Ca(OH)2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���ɣ���b�˹��塣
���з���C���ȵõ���ʦ�϶����ݸ÷���ʵ�����b��a���� ��ϵ����֤�������������εĻ�������Ϊ��������C�⣬�����ķ������� ��
��1��Na3N��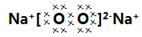
(2) 6Na+2Fe2O3=3Na2O2+4Fe
(3) 2N2O2+4CO2+2H2O=4NaHCO3+O2
��4��D
��5��53��84a�� b �� a B D
���������������1����������֪��Na��Fe��N��O����Ԫ����ɵĻ������в�����ˮ������������ĺ���ɫ����ֻ��Fe2O3��������Ԫ����ɵĵ����У��ڸ��¸�����������������Fe2O3��Ӧ��ֻ�н����ƣ����������ƣ�����ɫ�������Ϊ�������ƣ������������Na��NԪ����ɣ�13.0g������ף�����ʹ����ȫ�ֽ⣬���ɵ����ͽ����ƣ����ɵĵ����ۺϳɱ�״���µ����Ϊ6.72L����0.3mol��������֪Na��N��ԭ�Ӹ�������1��3��������Ļ�ѧʽΪNaN3���������Ƶĵ���ʽΪ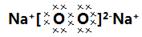 ����2�������������ɫ��ĩ������������Ӧ�Ļ�ѧ����ʽΪ6Na+2Fe2O3=3Na2O2+4Fe����3�����������ڿ�����ת��Ϊ̼�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ2N2O2+4CO2+2H2O=4NaHCO3+O2����4����ȫ��������������ĩ�����������IJ����Ļ��õ��ƣ�ѡ�����ܺ��Ʒ�Ӧ��������ͭ��ѡD����5���������Ƹ������Ӵ����õĿ����Ե��γɷֿ�����Na2CO3����NaHCO3����Na2CO3��NaHCO3����̼�������������ֽ⣬̼���������ѷֽ⣬�����ü��ȵİ취��ȷ���ɷ֣�C������ȡa�˻�����ּ��ȣ���b�˹��壬������Ϊ̼����ʱ�����ȣ����岻�ֽ⣬��Ӧǰ������������䣬a=b��������Ϊ̼������ʱ������2NaHCO3
����2�������������ɫ��ĩ������������Ӧ�Ļ�ѧ����ʽΪ6Na+2Fe2O3=3Na2O2+4Fe����3�����������ڿ�����ת��Ϊ̼�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ2N2O2+4CO2+2H2O=4NaHCO3+O2����4����ȫ��������������ĩ�����������IJ����Ļ��õ��ƣ�ѡ�����ܺ��Ʒ�Ӧ��������ͭ��ѡD����5���������Ƹ������Ӵ����õĿ����Ե��γɷֿ�����Na2CO3����NaHCO3����Na2CO3��NaHCO3����̼�������������ֽ⣬̼���������ѷֽ⣬�����ü��ȵİ취��ȷ���ɷ֣�C������ȡa�˻�����ּ��ȣ���b�˹��壬������Ϊ̼����ʱ�����ȣ����岻�ֽ⣬��Ӧǰ������������䣬a=b��������Ϊ̼������ʱ������2NaHCO3 Na2CO3 + CO2��+ H2O֪�������������٣�b=53��84a��������Ϊ���ߵĻ����ʱ��53��84a�� b �� a��A��ȡa�˻����������ϡ�����ַ�Ӧ���ݳ����������̼��ˮ�������ܱ���ʯ�����գ���ȷ����������ɣ�B��ȡa�˻����������ϡ�����ַ�Ӧ�����ȡ����ɡ����գ���b���Ȼ��ƹ��壬�з�������㣬��ȷ���������ɣ�D��ȡa�˻����������Ca(OH)2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���ɣ���b��̼��ƹ����з�������㣬��ȷ���������ɣ�ѡBD��
Na2CO3 + CO2��+ H2O֪�������������٣�b=53��84a��������Ϊ���ߵĻ����ʱ��53��84a�� b �� a��A��ȡa�˻����������ϡ�����ַ�Ӧ���ݳ����������̼��ˮ�������ܱ���ʯ�����գ���ȷ����������ɣ�B��ȡa�˻����������ϡ�����ַ�Ӧ�����ȡ����ɡ����գ���b���Ȼ��ƹ��壬�з�������㣬��ȷ���������ɣ�D��ȡa�˻����������Ca(OH)2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���ɣ���b��̼��ƹ����з�������㣬��ȷ���������ɣ�ѡBD��
���㣺���������ƶϡ��������㡢ʵ�鷽���ķ������ۡ�

 ȫ��������ϵ�д�
ȫ��������ϵ�д� һ��һ����ʱ���ϵ�д�
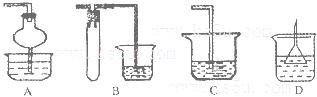
һ��һ����ʱ���ϵ�д����и�ͼ��ʾʵ������ܴﵽ��Ӧʵ��Ŀ�ĵ���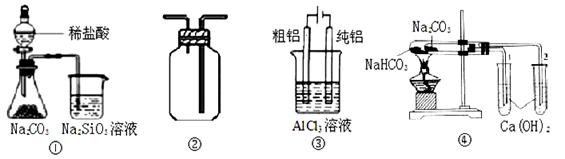
| A����ͼ��װ����֤�ȡ�̼����Ԫ�طǽ����� |
| B����ͼ��װ�����ռ�O2��CO2��H2 |
| C����ͼ��װ�õ�⾫���� |
| D����ͼ��װ����֤NaHCO3��Na2CO3�����ȶ��� |
����������ѧ�ڷϾɵ�ػ��մ�����״�о������б�����һ��ʪ�����������������ǶԷϾɵ�ز���(����Ni(OH)2��̼�ۡ���������������)������Դ��������Ƶ�ʵ������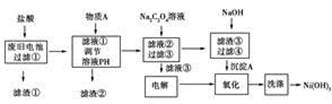
��֪����NiCl2������ˮ��Fe3����������Ni2����
����֪ʵ���¶�ʱ���ܽ�ȣ�NiC2O4��NiC2O4��H2O��NiC2O4��2H2O
�۽��������ڸ�ʵ����������ȫ������pH��
| ���� | Al3�� | Fe3�� | Ni2�� |
| pH | 5.2 | 4.1 | 9.7 |
(1)����A �������ǵ�����Һ��pH�Գ�ȥ����Fe3����Al3�����������ʺ���ΪA���ʵ���________��
A��NiO B������ C��NaOH D����ˮ
(2)�����ڵ���Ҫ�ɷ���_________________________________��
(3)д������Na2C2O4��Һ��Ӧ�Ļ�ѧ����ʽ�� _____________��
(4)д�������Һ�۵�������Ӧʽ___________________________��
��������Һ��ʱ��������������ķ�������ʪ���________��ֽ���顣
(5)�ɳ���A����Ni(OH)3�Ĺ����������������м������NaOH��Һ���г���ת��������Ӧ��ȫ����ͨ������Һ�۲��������������������д�����������̵����ӷ���ʽ��__________________��
(6)��μ���Ni(OH)3�Ƿ�ϴ�Ӹɾ���
________________________________��
(15��)ij�о�С���������װ�÷���CO��CO2�Ļ�����岢̽��CO��ԭ����ͭ�IJ���������Ƶ����ʡ���֪�Ȼ��٣�PdCl2����Һ��CO����������ɫ����(��������)���г�װ��δ������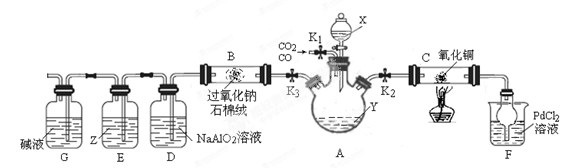
����9ͼ��
��1���Լ�X��Y����ɫ��Ӧ���ʻ�ɫ����X��Y������ �� .
��2������װ�á����������Բ�װ��ҩƷ�رշ�Һ©��������K3����K1��K2�������CO�����۲쵽 ����ʱ���ɶ�Cװ�ý��м��Ȳ�������Ӧ����Ϊ��ɫ���塣
��3����ѯ���ϻ��������Ϣ����Cu2OΪ��ɫ���壻�ڳ����£�Cu2+����Һ���ȶ���Cu+�������������·�����Ӧ��2Cu+ =Cu2++Cu��
�ס�������ͬѧȡ������ɫ����(��M����)����ʵ�飬̽����ɷ֣�
| ��� | ʵ����� | ʵ����������� | �� �� |
| �� | �� ��a g M�м���һ����ϡ���ᣬ��ֽ��裻 �� �����μ�ϡ����������, ��ַ�Ӧ. | �ٹ������Լ��٣� ����Ȼ��һ�������壬��Һ����ɫ | ��M��һ����Cu2O; ��M��һ����Cu. |
| �� | ����ʵ���������Һ���� ������ϴ�ӡ�������� | ��������Ϊ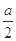 g g | MΪCu��Cu2O�Ļ���� |
(ii)����ͬѧ�������ͬѧ�����˼���,��Ϊ����ͬѧ�Ľ�������ȷ��,���ó�M��Cu��Cu2O�����ʵ���֮���� .
��4��Cװ�÷�Ӧ���������ӻ�������з����CO2������е���Ҫ������ .ʵ�������Dװ�ò�����ɫ���������ӷ���ʽΪ .
��5��Z�Լ�Ϊ����KI�ı���Һ����Eװ���п��ܲ����������� .

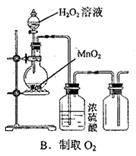
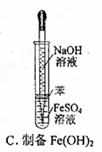
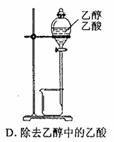
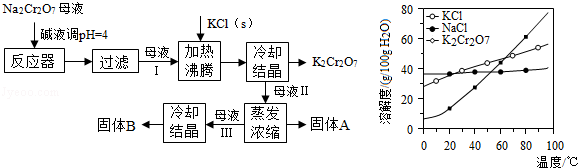
 2CrO42������ɫ����2H+
2CrO42������ɫ����2H+