ЬтФПФкШн
ЁОЬтФПЁПФГаЫШЄаЁзщЕФЭЌбЇЩшМЦЪЕбщжЦБИCuBr(АзЩЋНсОЇадЗлФЉЃЌЮЂШмгкЫЎЃЌВЛШмгкввДМЕШгаЛњШмМС)ЃЌЪЕбщзАжУ(МаГжЁЂМгШШвЧЦїТд)ШчЭМЫљЪОЁЃ
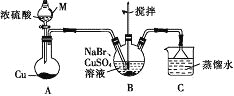
(1)вЧЦїMЕФУћГЦЪЧ________ЁЃ
(2)ШєНЋMжаЕФХЈСђЫсЛЛГЩ70%ЕФH2SO4ЃЌдђдВЕзЩеЦПжаЕФЙЬЬхЪдМСЮЊ______(ЬюЛЏбЇЪН)ЁЃ
(3)BжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_______ЃЌФмЫЕУїBжаЗДгІвбЭъГЩЕФвРОнЪЧ_____ЁЃШєBжаCu2+ШдЮДЭъШЋБЛЛЙдЃЌЪЪвЫМгШыЕФЪдМСЪЧ_______(ЬюБъКХ)ЁЃ
a.вКфх b.Na2SO4 c.ЬњЗл d.Na2S2O3
(4)ЯТСаЙигкЙ§ТЫЕФа№ЪіВЛе§ШЗЕФЪЧ_______ (ЬюБъКХ)ЁЃ
a.ТЉЖЗФЉЖЫОБМтПЩвдВЛНєППЩеББк
b.НЋТЫжНШѓЪЊЃЌЪЙЦфНєЬљТЉЖЗФкБк
c.ТЫжНБпдЕПЩвдИпГіТЉЖЗПк
d.гУВЃСЇАєдкТЉЖЗжаЧсЧсНСЖЏвдМгПьЙ§ТЫЫйТЪ
(5)ЯДЕгЪБЃЌЯШгУзАжУCжаЕФЮќЪевКЧхЯДЃЌЦфФПЕФЪЧ_______ЃЌдйвРДЮгУШмНтSO2ЕФввДМЁЂввУбЯДЕгЕФФПЕФЪЧ________ЁЃ
ЁОД№АИЁПЗжвКТЉЖЗ Na2SO3(ЛђK2SO3ЛђNaHSO3ЛђKHSO3ЕШ) 2CuSO4+2NaBr+SO2+2H2O=2CuBrЁ§+Na2SO4+2H2SO4 ШмвКРЖЩЋЭЪШЅ d acd ЗРжЙCuBrБЛбѕЛЏ гУввДМГ§ШЅЙЬЬхБэУцЕФЫЎЃЌдйгУИќвзЛгЗЂЕФввУбГ§ШЅввДМЃЌЪЙЦфПьЫйИЩдя
ЁОНтЮіЁП
(1)ИљОнвЧЦїНсЙЙХаЖЯвЧЦїЕФУћГЦЃЛ
(2)ИљОнИДЗжНтЗДгІЕФЙцТЩбЁдёЪдМСЃЛ
(3)дкBжаNaBrЁЂSO2ЁЂCuSO4ЁЂH2OЛсЗЂЩњбѕЛЏЛЙдЗДгІВњЩњCuBrГСЕэЃЌРћгУЕчзгЪиКуЁЂдзгЪиКуЃЌЪщаДЗДгІЕФЛЏбЇЗНГЬЪНЃЌИљОнCu2+ЕФЫЎШмвКЯдРЖЩЋЃЌНсКЯИУЗДгІЕФЬиЕуХаЖЯЗДгІЭъШЋЕФЬиеїМАМгШыЕФЮяжЪЃЛ
(4)ИљОнЙ§ТЫВйзїЕФФПЕФЁЂвЧЦїЕФЪЙгУЗНЗЈНтД№ЃЛ
(5)CuBrШнвзБЛбѕЛЏЃЌИљОнSO2ЕФЫЎШмвКОпгаЛЙдадЗжЮіЃЌНсКЯH2OШнвзШмгкввДМжаЃЌМАввДМЁЂввУбОпгавзЛгЗЂЕФаджЪЗжЮіЁЃ
(1)ИљОнзАжУЭМжавЧЦїMЕФНсЙЙПЩжЊИУвЧЦїЕФУћГЦЪЧЗжвКТЉЖЗЃЛ
(2)ИљОнИДЗжНтЗДгІЕФЙцТЩПЩгУ70%ЕФH2SO4гыNa2SO3ЛђK2SO3ЛђNaHSO3ЛђKHSO3ЕШЗДгІжЦШЁSO2ЦјЬхЃЌЗДгІЗНГЬЪНЮЊH2SO4+Na2SO3=Na2SO4+H2O+SO2ЁќЃЛ
(3)дкBжаBaBrЁЂSO2ЁЂCuSO4ЁЂH2OЛсЗЂЩњбѕЛЏЛЙдЗДгІВњЩњCuBrГСЕэЃЌРћгУЕчзгЪиКуЁЂдзгЪиКуЃЌПЩЕУИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊЃК2CuSO4+2NaBr+SO2+2H2O=2CuBrЁ§+Na2SO4+2H2SO4ЃЛ
Cu2+ЕФЫЎШмвКЯдРЖЩЋЃЌШєЗДгІЭъШЋЃЌдђШмвКжаВЛдйКЌгаCu2+ЃЌШмвКЕФРЖЩЋЭЪШЅЃЌгЩгкCuSO4ЪЧЧПЫсШѕМюбЮЃЌдкШмвКжаCu2+ЫЎНтЪЙШмвКЯдЫсадЃЌH+гыNa2S2O3ЗДгІВњЩњSO2ЦјЬхЃЌвдДйЪЙЗДгІЕФНјааЃЌвђДЫШєBжаCu2+ШдЮДЭъШЋБЛЛЙдЃЌЪЪвЫМгШыЕФЪдМСЪЧNa2S2O3ЃЌЙЪКЯРэбЁЯюЪЧdЃЛ
(4)a.ТЉЖЗФЉЖЫОБМтНєППЩеББкЃЌОЭПЩвдЪЙЙ§ТЫЕУЕНЕФТЫвКбиЩеБФкБкВЛЖЯНјШыЕНЩеБжаЃЌaДэЮѓЃЛ
b.НЋТЫжНШѓЪЊЃЌЪЙЦфНєЬљТЉЖЗФкБкЃЌОЭПЩвдЪЙЛьКЯЮяГфЗжЗжРыЃЌbе§ШЗЃЛ
c.ЮЊСЫЪЙФбШмадЕФЙЬЬхгывКЬхЮяжЪЗжРыЃЌТЫжНБпдЕвЊЕЭгкТЉЖЗПкБпдЕЃЌcДэЮѓЃЛ
d.гУВЃСЇАєв§СїЃЌЪЙЛьКЯЮяНјШыЕНЙ§ТЫЦїжаЃЌдкТЉЖЗжаВЛФмгУВЃСЇАєНСЖЏЃЌЗёдђЛсЪЙТЫжНЦЦЫ№ЃЌЕМжТВЛФмЙ§ТЫЃЌВЛФмЗжРыЛьКЯЮяЃЌdДэЮѓЃЛ
ЙЪКЯРэбЁЯюЪЧacdЃЛ
(5)SO2ЪЧДѓЦјЮлШОЮяЃЌЮЊЗРжЙЦфЮлШОЛЗОГЃЌгУеєСѓЫЎЮќЪеSO2ЃЌЕУЕНH2SO3ШмвКЃЌИУЮяжЪОпгаЛЙдадЃЌгУH2SO3ШмвКЯДЕгЃЌОЭПЩвдБмУтCuBrБЛПеЦјжаЕФбѕЦјбѕЛЏЃЌШЛКѓвРДЮгУШмНтSO2ЕФввДМЁЂввУбЯДЕгЕФФПЕФЪЧгУввДМГ§ШЅЙЬЬхБэУцЕФЫЎЃЌдйгУИќвзЛгЗЂЕФввУбГ§ШЅввДМЃЌЪЙЦфПьЫйИЩдяЁЃ

 УћаЃПЮЬУЯЕСаД№АИ
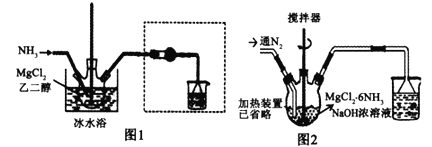
УћаЃПЮЬУЯЕСаД№АИЁОЬтФПЁПвбжЊMgCl2+6NH3![]() MgCl2ЁЄ6NH3ИУЗДгІОпгаМЋКУЕФПЩФцЮќЁЂЗХАБЬиадЁЃФГПЮЬтзщдкЪЕбщЪвЬНОПЦфЬиадЃЌЦфжаАБЛЏ(ЮќАБ)Й§ГЬЪЕбщзАжУШчЭМ1ЃЌЗХАБЙ§ГЬЪЕбщзАжУШчЭМ2ЁЃ
MgCl2ЁЄ6NH3ИУЗДгІОпгаМЋКУЕФПЩФцЮќЁЂЗХАБЬиадЁЃФГПЮЬтзщдкЪЕбщЪвЬНОПЦфЬиадЃЌЦфжаАБЛЏ(ЮќАБ)Й§ГЬЪЕбщзАжУШчЭМ1ЃЌЗХАБЙ§ГЬЪЕбщзАжУШчЭМ2ЁЃ
МКжЊЃКЯрЙиЮяжЪЕФаджЪМћЯТБэ
ЮяжЪУћГЦ | АБЦјЕФШмНтад | ТШЛЏУОЕФШмНтад | СљАБТШЛЏУОЕФШмНтад |
ЫЎ | взШм | взШм | взШм |
МзДМЃЈЗаЕу65ЁЃCЃЉ | взШм | взШм | ФбШм |
ввЖўДМЃЈЗаЕу197ЁЃCЃЉ | взШм | взШм | ФбШм |
ЧыЛиД№ЃК
ЃЈ1ЃЉЪЕбщЪвВЩгУЙЬЙЬМгШШЕФЗНЪНжЦБИNH3ЃЌжЦБИЗДгІЕФЗНГЬЪНЮЊ___________ЃЌащПђФкзАжУЕФзїгУЪЧ___________ЃЌАБЛЏЙ§ГЬВЩгУБљЫЎдЁЕФдвђПЩФмЪЧ_____(ЬюбЁЯюађКХзжФИ)ЁЃ
AЃЎАБЛЏЙ§ГЬЮЊЮќШШЗДгІЃЌДйНјЗДгІе§ЯђНјаа
BЃЎМгПьЗДгІЫйТЪ
CЃЎЗРжЙАБЦјДгШмвКжаЛгЗЂЃЌЬсИпАБЦјРћгУТЪ
DЃЎдіДѓСљАБТШЛЏУОЕФШмНтЖШ
ЃЈ2ЃЉРћгУMgCl2ШмвКжЦБИЮоЫЎMgCl2ЃЌЦфОпЬхВйзїЮЊ______________________ЁЃ
ЃЈ3ЃЉНјааЗХАБЪЕбщЪБЃЌШ§ОБЩеЦПМгШы1.97 g MgCl2ЁЄ6NH3КЭЩеМюЕФХЈШмвКЃЌМгШШЃЌВЂВЛЖЯЭЈШыN2ЃЌЭЈШыN2ФПЕФЪЧ___________ЁЃЭЈЙ§ЙлВьЩеБжаЯжЯѓЕФБфЛЏОЭПЩвдМрПиMgCl2ЁЄ6NH3ЕФЗХАБЙ§ГЬЃЌШєвЊХаЖЯзЊЛЏТЪЪЧЗёДяЕНЛђГЌЙ§90%ЃЌдђЩеБжаШмвКПЩвдЪЧ___________ЁЃ