��Ŀ����
����Ŀ����ͼ�Dz�������Ԫ�����ڱ���A��H����Ԫ�ص�λ����ȷ����������и��⡣
A | |||||||||||||||||
B | C | D | |||||||||||||||
E | F | G | |||||||||||||||
H | |||||||||||||||||
��1����ʵ����Ԫ�����ڱ����ϱ߽硣����Ӱ��ʾ���ǽ���Ԫ�ء������ú�ɫˮ����д��__________
��2����һ���û���Ӧ֤��F��G�ķǽ�����ǿ��������д���ӷ���ʽ��_____________��
��3����֪A��C���γɺ���18�����ӵĻ�����京�еĻ�ѧ��������Ϊ__________��__________��
��4����֪A��CҲ���γ�CA5�����ӻ��������д�����ʽ__________________��
��5��H����C������������Ӧ��ˮ�����ϡ��Һ��Ӧ��д����Ӧ�����ӷ���ʽ________________��
��6���ж�B���⻯���E���⻯��ķе�ߵͣ�BHm_____________EHm������ڡ��������ڡ���С�ڡ�����
��7��C���⻯�K������D���⻯���ԭ����_____________________________��
���𰸡� 
�����ϱ߽�1�֣������ǽ�������Ӱ��ע�����Ͻǵ��⣩ Cl2 + H2S = S�� + 2H+ + 2Cl- ���� Cl2 + S2- = S�� + 2Cl- �Ǽ��Լ� ���Լ� ![]() 3Cu + 8H++ 2NO3- = 3Cu2++2NO��+ 4 H2O С��
3Cu + 8H++ 2NO3- = 3Cu2++2NO��+ 4 H2O С�� ![]() NH3��H2O���Ǽ��Է��ӡ�
NH3��H2O���Ǽ��Է��ӡ� ![]() NH3��H2O֮���γ������
NH3��H2O֮���γ������
![]() NH3��H2O֮����Է�Ӧ��
NH3��H2O֮����Է�Ӧ��
������������Ԫ�����ڱ��Ľṹ��Ԫ�صķֲ�������֪��AΪH��BΪC��CΪN��DΪO��EΪSi��FΪS��GΪCl��HΪCu��
��1��Ԫ�����ڱ��Ľṹ�Լ�Ԫ�صķֲ��������ʾԪ�����ڱ����ϱ߽缰�ǽ���Ԫ�����£�
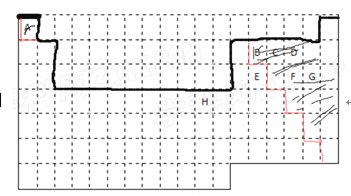
��2��S��Cl�ķǽ�����ǿ��������Cl2 + H2S = S�� + 2H+ + 2Cl- ���� Cl2 + S2- = S�� + 2Cl-����3��H��N���γɺ���18�����ӵĻ�����N2H4���京�еĻ�ѧ��������Ϊ���Թ��ۼ��ͷǼ��Թ��ۼ�����4��H��N�γɵ����ӻ�����NH4H�������ʽΪ��![]() ����5��Cu��N������������Ӧ��ˮ����HNO3��ϡ��Һ��Ӧ�����ӷ���ʽΪ��3Cu + 8H++ 2NO3- = 3Cu2++2NO��+ 4 H2O����6��ͬ����Ԫ�ص��⻯�������ɺͽṹ���ƣ�һ����Է�������Խ��,���Ӽ�������Խǿ���е�Խ�ߣ���C���⻯���Si���⻯��ķе�ߵͣ�CHm<SiHm����7����NH3��H2O���Ǽ��Է��ӣ���NH3��H2O֮���γ��������NH3��H2O֮����Է�Ӧ����һˮ�ϰ�����NH3��������H2O��
����5��Cu��N������������Ӧ��ˮ����HNO3��ϡ��Һ��Ӧ�����ӷ���ʽΪ��3Cu + 8H++ 2NO3- = 3Cu2++2NO��+ 4 H2O����6��ͬ����Ԫ�ص��⻯�������ɺͽṹ���ƣ�һ����Է�������Խ��,���Ӽ�������Խǿ���е�Խ�ߣ���C���⻯���Si���⻯��ķе�ߵͣ�CHm<SiHm����7����NH3��H2O���Ǽ��Է��ӣ���NH3��H2O֮���γ��������NH3��H2O֮����Է�Ӧ����һˮ�ϰ�����NH3��������H2O��



