��Ŀ����
����A��B��C��D��E����Ԫ�أ����ڶ�����Ԫ�أ��й���Ϣ�����ʾ��
��1��AԪ������Ϊ ��
��2��B3A�ĵ���ʽΪ ��
��3����DC3��Һ���ȡ��������տɵõ��������� ��
��4��д����84����Һ�����ӷ���ʽ ��
| ԭ�ӻ���Ӳ��ֽṹ֪ʶ | ���ʻ���IJ���������; | |||
| A | A����̬�⻯��ռ�ṹ�������� | ����������ˮ������ˮ����ȫ���� | ||
| B | Bԭ�ӵ��������������ڵ��Ӳ�����
|
B3A�����ӻ����� | ||
| C | Cԭ�ӵ��������������ڣ�2n+1����n���Ӳ����� | �������ռ���Һ��Ӧ����84����Һ | ||
| D | Dԭ����ʧȥ3�����Ӵﵽ�ȶ��ṹ | ���ʿ���Ϊұ��ҵ�Ļ�ԭ�� | ||
| E | Eԭ��������������K���������3�� | �γɵĵ��ʼȾ����������־��л�ԭ�� |
��2��B3A�ĵ���ʽΪ
��3����DC3��Һ���ȡ��������տɵõ���������
��4��д����84����Һ�����ӷ���ʽ
������A��B��C��D��E���ֶ�����Ԫ�أ�A����̬�⻯��ռ�ṹ�������Σ����ڢ�A�壬����������ˮ������ˮ����ȫ���룬����ǿ�ᣬ��AΪ��Ԫ�أ�Bԭ�ӵ��������������ڵ��Ӳ�����
����B���ڵ������ڣ�����������Ϊ1����BΪNaԪ�أ�Cԭ�ӵ��������������ڣ�2n+1����n���Ӳ��������������ռ���Һ��Ӧ����84����Һ����CΪClԪ�أ�Dԭ����ʧȥ3�����Ӵﵽ�ȶ��ṹ�����ʿ���Ϊұ��ҵ�Ļ�ԭ������DΪAlԪ�أ�Eԭ��������������K���������3��������������Ϊ6���γɵĵ��ʼȾ����������־��л�ԭ�ԣ���EΪ��Ԫ�أ��ݴ˽��
| 1 |
| 3 |
����⣺A��B��C��D��E���ֶ�����Ԫ�أ�A����̬�⻯��ռ�ṹ�������Σ����ڢ�A�壬����������ˮ������ˮ����ȫ���룬����ǿ�ᣬ��AΪ��Ԫ�أ�Bԭ�ӵ��������������ڵ��Ӳ�����
����B���ڵ������ڣ�����������Ϊ1����BΪNaԪ�أ�Cԭ�ӵ��������������ڣ�2n+1����n���Ӳ��������������ռ���Һ��Ӧ����84����Һ����CΪClԪ�أ�Dԭ����ʧȥ3�����Ӵﵽ�ȶ��ṹ�����ʿ���Ϊұ��ҵ�Ļ�ԭ������DΪAlԪ�أ�Eԭ��������������K���������3��������������Ϊ6���γɵĵ��ʼȾ����������־��л�ԭ�ԣ���EΪ��Ԫ�أ�
��1��������������֪��AΪ��Ԫ�أ��ʴ�Ϊ������
��2��B3AΪNa3N���������ӻ�����������ʽΪ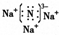 ���ʴ�Ϊ��
���ʴ�Ϊ��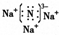 ��
��
��3��AlCl3��Һ�д���ƽ��AlCl3+3H2O?Al��OH��3+HCl����Һ����HCl�ӷ����ٽ�ˮ�⳹���У��õ�Al��OH��3������Al��OH��3�ֽ�������������ˮ�����տɵõ���������Al2O3���ʴ�Ϊ��Al2O3��
��4����84����Һ�����ӷ���ʽΪCl2+2OH-?ClO-+Cl-+H2O���ʴ�Ϊ��Cl2+2OH-?ClO-+Cl-+H2O��
| 1 |
| 3 |
��1��������������֪��AΪ��Ԫ�أ��ʴ�Ϊ������
��2��B3AΪNa3N���������ӻ�����������ʽΪ
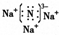 ���ʴ�Ϊ��
���ʴ�Ϊ��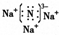 ��
����3��AlCl3��Һ�д���ƽ��AlCl3+3H2O?Al��OH��3+HCl����Һ����HCl�ӷ����ٽ�ˮ�⳹���У��õ�Al��OH��3������Al��OH��3�ֽ�������������ˮ�����տɵõ���������Al2O3���ʴ�Ϊ��Al2O3��
��4����84����Һ�����ӷ���ʽΪCl2+2OH-?ClO-+Cl-+H2O���ʴ�Ϊ��Cl2+2OH-?ClO-+Cl-+H2O��
���������⿼��ṹ����λ�ù�ϵӦ�ã��ѶȲ����ضԳ��û�ѧ����Ŀ��飬�ƶ�Ԫ���ǹؼ�����3��ע���������ˮ����з������ע�����֪ʶ�����գ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��






