��Ŀ����
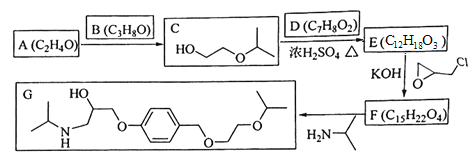
����Ŀ��������G�����Ƹ�Ѫѹ��ҩ�������������м��壬һ�ֺϳ�G��·�����£�
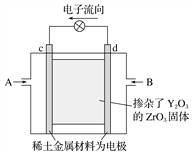
��֪����A�˴Ź�������Ϊ���壻B�ĺ˴Ź�������Ϊ����壬�������Ϊ6��1��1��
��D�ı����Ͻ������ֲ�ͬ��ѧ�������⣻1 mol D����1 mol NaOH��2 mol Na��Ӧ��
�ش��������⣺
��1��A����C�Ļ�ѧ����ʽΪ____________________________��
��2��C�Ĺ���������Ϊ____________��
��3��D�Ľṹ��ʽΪ_________________��
��4����E����F�ķ�Ӧ����Ϊ____________��
��5��L��D��ͬ���칹�壬����FeCl3��Һ������ɫ��Ӧ��1 mol��L����1 mol��Na2CO3��Ӧ��L�˴Ź�������Ϊ����壬�������Ϊ3��2��2��1��L�Ľṹ��ʽΪ___________��
���𰸡� ![]() �ǻ� �Ѽ�
�ǻ� �Ѽ� ![]() ȡ����Ӧ
ȡ����Ӧ ![]()
�����������⿼���л���Ľṹ�����ʵ��ƶϡ��漰�����ŵ����ơ���ѧ����ʽ��ͬ���칹�����д�ȡ�
��1��A�Ļ�ѧʽΪC2H4O����˴Ź�������Ϊ���壬��AΪ![]() ��B�Ļ�ѧʽΪC3H8O���˴Ź�������Ϊ����壬�������Ϊ6��1��1����B�Ľṹ��ʽΪCH3CH(OH)CH3����A����C�Ļ�ѧ����ʽΪ
��B�Ļ�ѧʽΪC3H8O���˴Ź�������Ϊ����壬�������Ϊ6��1��1����B�Ľṹ��ʽΪCH3CH(OH)CH3����A����C�Ļ�ѧ����ʽΪ![]() ��
��
��2����C�Ľṹ��ʽ��֪C�к��еĹ�����Ϊ�ǻ����Ѽ���
��3��D�Ļ�ѧʽΪC7H8O2���䱽���Ͻ������ֲ�ͬ��ѧ�������⣬1 mol D����1 mol NaOH��2 mol Na��Ӧ�������з��ǻ�����CH2OH����Ϊ��λ�ṹ����D�Ľṹ��ʽΪ ![]() ��
��
��4��![]() ��
��![]() ����ȡ����Ӧ����F��
����ȡ����Ӧ����F��
��5��L��![]() ��ͬ���칹�壬����FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ���1 mol��L����1 mol��Na2CO3��Ӧ��˵��L�����к���1�����ǻ���L�˴Ź�������Ϊ����壬�ҷ������Ϊ3��2��2��1�Ľṹ��ʽΪ
��ͬ���칹�壬����FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ���1 mol��L����1 mol��Na2CO3��Ӧ��˵��L�����к���1�����ǻ���L�˴Ź�������Ϊ����壬�ҷ������Ϊ3��2��2��1�Ľṹ��ʽΪ![]() ��
��

 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�