��Ŀ����
����Ŀ���п�Ժ��������������ϸ������ij����ַ�������ͼ��ʾ��


��1��������ͼ��Ϣ���Կ��������е�������Ⱦ�ﲢ���ɻ�������ʻ��ɵ���______����������������ϡȼ����ϵͳ��Ҫ����ԭ��������ͼ��ʾ��д��ϡȼ������NO��������Ҫ��Ӧ�ķ���ʽ_____________________________________��
��2��ũҵ��ų��İ�������ʩ�õĻ��ʷֽ⣬Ҳ������ʩ�ò������µġ�����ijЩ��������Է��ϻ��ʩ�û��ͳ����������ӷ���ʽ����________________________��
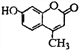
��3�������о������ҹ����������ԣ�����Ҫԭ������ͼ��ʾ��A�Ļ�ѧʽ��________��
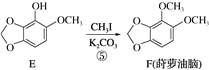
2NH3(��)+SO2(��)+2NO2(��)![]() 2NH4+(��Һ)+A(��Һ)+2HONO(��)
2NH4+(��Һ)+A(��Һ)+2HONO(��)
��4��úȼ���ŷŵ���������SO2��NOx������NaClO2��Һ��Ϊ���ռ���ͬʱ��������������������������SO2��NOx������ͨ��ʢ��NaClO2��Һ�ķ�Ӧ���У���Ӧһ��ʱ������Һ������Ũ�ȵ��й��������£������������Ӻ��Բ��ƣ���
���� | Na+ | SO42�� | NO���� | H+ | Cl |
Ũ��/��mol��L1�� | 5.5��103 | 8.5��104 | y | 2.8��104 | 3.5��103 |
��NO��NaClO2��Һ��Ӧ�����ӷ���ʽ��___________________��
�ڱ���y��_______��
��5����ҵ��������Ҳ�п��ܲ���NOx��Ⱦ����д�����������еĵ�һ�������Ĵ������Ļ�ѧ����ʽ___________________________________�����õ����ư��IJ���Ϊ90%���ð���������ʱ���Ĵ������͵�������ת��Ϊ������������Ϊ5%��3%��1000 mol��������___________mol���ᡣ
���𰸡�SO22NO+O2��2NO2NH4��+OH-��NH3��+H2OSO42��4NO+3ClO2��+2H2O��4NO3��+3Cl-+4H+5.8��1044NH3+5O2![]() 4NO+6H2O1659
4NO+6H2O1659
��������
��1��������ͼ��Ϣ���Կ��������е�������Ⱦ�ﲢ���ɻ�������ʻ��ɵ���SO2��NO����������ΪNO2����ϡȼ������NO��������Ҫ��Ӧ�ķ���ʽΪ2NO+O2��2NO2��
��2�������ǿ�Ӧ��ų�������������Է��ϻ��ʩ�û��ͳ��������ӷ���ʽΪNH4��+OH-��NH3��+H2O��
��3������ԭ���غ�͵���غ��֪A�Ļ�ѧʽ��SO42����
��4����NO��NaClO2��Һ����������ԭ��Ӧ����������������ӣ���Ӧ�����ӷ���ʽ��4NO+3ClO2��+2H2O��4NO3��+3Cl-+4H+��
�ڸ��ݵ���غ��֪����y��(5.5��10��3+2.8��10��4�D3.5��10��3�D2��8.5��10��4)mol��L��1��5.8��10��4 mol��L��1��
��5�����Ĵ������Ļ�ѧ����ʽΪ4NH3+5O2![]() 4NO+6H2O�����ݵ�ԭ���غ��֪1000 mol�������Ƶ���������ʵ�����1000mol��2��0.9��0.95��0.97��1659mol��
4NO+6H2O�����ݵ�ԭ���غ��֪1000 mol�������Ƶ���������ʵ�����1000mol��2��0.9��0.95��0.97��1659mol��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ���Ҵ�������ϩ����ֱ��ˮ�Ϸ�����ˮ�Ϸ�������һЩת����ͼ��

ijЩ���ʵ��й��������±���
�۵�/�� | �е�/�� | ˮ���� | |
�Ҵ� | -114.1 | 78.3 | ���� |
��ȩ | -121 | 20.8 | ���� |
�������� | -83 | 77.0 | ���� |
�ش��������⣺
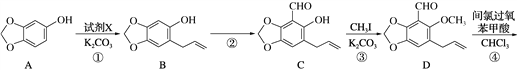
��1�����ˮ�Ϸ��е�ת���ۣ���ϩ��Ũ���ᷴӦ��������������(CH3CH2��OSO3H)���л���Ӧ������_____________��ת���ܵĻ�ѧ����ʽ��__________________��
��2��պ��B�IJ�����������������⻯�������ð���̣���Ӧ�Ļ�ѧ����ʽΪ________��ת���ݵĻ�ѧ����ʽΪ______________________________��
��3����һ����Ϊw��ͭ˿���ձ�ں�Ѹ�ٲ����Ҵ��У���ڵ�ͭ˿�ָ���ɫ��
��ʹͭ˿�ָ���ɫ�ķ�Ӧ�Ļ�ѧ����ʽΪ________________________��
����Ҫ֤��ͭ˿������ã�����Ҫ���еIJ�����_______________��
��4����֪��CH3CHO + NaHSO3 ��![]() ���������ǻ��һ����ƣ�������������ȩ�������·�ʽ�ᴿ��
���������ǻ��һ����ƣ�������������ȩ�������·�ʽ�ᴿ��
![]()
�٦����ǻ��һ����Ƶľ�������Ϊ___________________��
�ڷ������A��������________________��
��ijͬѧ��Ʒ������B��װ�ã��гֺͼ���װ������ȥ����ͼ��ʾ���������е�Һ��Ӧ��_____�ڽ������D����E����

�����йز�����װ�õķ�������ȷ����_____��������ĸ����ѡ���ۣ�
A���ձ���Ӧװ��ˮ
B��������Ӧͨ��ˮ
C��ͼʾװ�ÿ����ڳ�ȥ���������л��е��Ҵ�