��Ŀ����
��֪��Ȳ�뱽������ȫȼ�յ��Ȼ�ѧ����ʽ������ʾ��
��C2H2��g��+5/2O2��g����2CO2��g��+H2O��1�� ��H=��1300KJ/mol
��C6H6��g��+15/2O2��g����6CO2��g��+3H2O��1����H=��3295Kj/mol
����˵����ȷ���� �� ��
��C2H2��g��+5/2O2��g����2CO2��g��+H2O��1�� ��H=��1300KJ/mol
��C6H6��g��+15/2O2��g����6CO2��g��+3H2O��1����H=��3295Kj/mol
����˵����ȷ���� �� ��
| A��1mol C2H2��g����ȫȼ��������̬ˮʱ���ȴ���1300kJ |
| B��1mol C6H6��1����ȫȼ������Һ̬ˮʱ���ȴ���3295kJ |
| C����ͬ�����£���������C2H2��g����C6H6��g����ȫȼ�գ�C6H6��g�����ȸ��� |
| D��C2H2��g����������C6H6��g���Ĺ������ڷ��ȷ�Ӧ |
D
���������A������̬ˮ��ΪҺ̬ˮ��ų���������1mol C2H2��g����ȫȼ��������̬ˮʱ����С��1300kJ���ʴ���
B��C6H6��1����ΪC6H6��g��Ҫ������������1mol C6H6��1����ȫȼ������Һ̬ˮʱ����С��3295kJ���ʴ���
C������������Ϊmg����C2H2��g����C6H6��g�����ʵ����ֱ�Ϊ(m/26)mol��(m/78)mol���ֱ������Ե��Ȼ�ѧ����ʽ�У��ų��������ֱ�Ϊ50 KJ/mol��42 KJ/mol����C2H2��g�����ȸ��࣬�ʴ���
��ѡD��
���������⿼������Ȼ�ѧ����ʽ��Ӧ�ã���Ŀ�ѶȲ�����Ҫ����ѧ���Ի���֪ʶ�����ճ̶ȡ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 2NH3(g) ��H<0��ijʵ�齫3.0 mol N2(g)��4. 0 mol H2(g)�����ݻ�Ϊ10L���ܱ������У����¶�T1�·�Ӧ�����H2�����ʵ����淴Ӧʱ��ı仯����ͼ��ʾ��
2NH3(g) ��H<0��ijʵ�齫3.0 mol N2(g)��4. 0 mol H2(g)�����ݻ�Ϊ10L���ܱ������У����¶�T1�·�Ӧ�����H2�����ʵ����淴Ӧʱ��ı仯����ͼ��ʾ��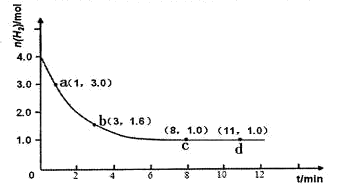

 O2(g)=SO2(g)+H2O(g) ��H1
O2(g)=SO2(g)+H2O(g) ��H1 O2(g)=S(g)+H2O(g) ��H3
O2(g)=S(g)+H2O(g) ��H3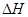 �ľ���ֵ����ȷ��
�ľ���ֵ����ȷ�� ��
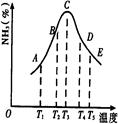
�� ��Ӧ�����������仯������ͼ������ͼ���ж�������������ȷ����
��Ӧ�����������仯������ͼ������ͼ���ж�������������ȷ����
 2NH3(g)��H=+92kJ/mol
2NH3(g)��H=+92kJ/mol �����Ǽ������ʱ�������仯����
�����Ǽ������ʱ�������仯���� N2(g)+3H2(g) ��H=+92kJ/mol
N2(g)+3H2(g) ��H=+92kJ/mol 2NH3�����ֱ���t��ʱ�ⶨ����NH3�������������ͼ���ң�
2NH3�����ֱ���t��ʱ�ⶨ����NH3�������������ͼ���ң�