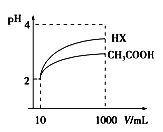
��Ŀ����
����Ŀ��ClO2��һ�������������������ܽ��Լ��Cl2��5�������¶ȹ���Ũ�ȹ���ʱ�������ֽ⣬��˳������Ƴ�KClO2���壬�Ա���������档�Ʊ�KClO2�����ʵ��װ����ͼ��ʾ������Aװ���Ʊ�ClO2��Bװ���Ʊ�KClO2����ش��������⣺
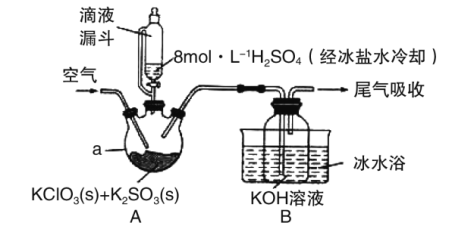
��1��A���Ʊ�ClO2�Ļ�ѧ����ʽΪ__��
��2�����Һ©����ȣ���ʵ��ʹ�õ�Һ©�������ŵ���__������H2SO4���ñ���ˮ��ȴ����Ϊ�˷�ֹҺ��ɽ���__��
��3��ʵ�������ͨ�������Ŀ����__���������ٹ��죬�ή��KClO2���ʣ��Խ�����ԭ��__��
��4��ClO2ͨ��KOH��Һ����KClO2��ͬʱ���п������ɵ�����__��
a.KCl b.KClO c.KClO3 d.KClO4
��5��KClO2���ʷֽ�ΪKClO3��KCl��ȡ�������ı���ǰ���KClO2���������Һ���ֱ���������FeSO4��Һ��Ӧ����Fe2+�����ʵ���__�����ͬ����������ͬ������ȷ��������
���𰸡�2KClO3+K2SO3+H2SO4=2K2SO4+2ClO2��+H2O ��Һ©��ʹ©����������ƿ�е�ѹǿ��ͬ��ʹҺ��˳������ ���ͷ�Ӧ��ϵ���¶ȣ���ֹ�¶ȸ�ClO2�ֽ� ��ClO2ȫ���ϳ��Ա�KOH��Һ���գ�ͬʱ��ϡ��ClO2����ֹŨ�ȹ������ֽ� �������ٹ��죬ClO2���ܱ�������� cd ��ͬ
��������
(1)A��KClO3��K2SO3��H2SO4��Ӧ�Ʊ�ClO2��KClO3�õ��ӣ�����ԭ����K2SO3������������K2SO4�����ݵ�ʧ�����غ㡢Ԫ���غ���ƽ��ѧ����ʽ��
(2)���������Ľṹ������Һ©�������ƣ�������֪��Ϣ��ClO2�¶ȹ���ʱ�����ֽ���
(3)�����ɽ�ClO2ȫ���ϳ��Ա�KOH��Һ���գ�ͬʱ����ClO2Ũ�ȹ���ʱ�����ֽ⣬���������ã�
(4)������ԭ��Ӧ�У����ϼ��н��ͣ��ض������ߣ�
(5)KClO2���ʷֽ�ΪKClO3��KCl�����ʷ����ķ�ӦΪ��3KClO2=2KClO3+KCl����KClO2��KClO3����Fe2+��ԭΪCl-�����ݵ���ת���غ�����жϡ�
(1)A��KClO3��K2SO3��H2SO4��Ӧ�Ʊ�ClO2��KClO3�õ��ӣ�����ԭ����K2SO3������������K2SO4�����ݵ�ʧ�����غ㡢Ԫ���غ���ƽ��ѧ����ʽΪ2KClO3+K2SO3+H2SO4=2K2SO4+2ClO2��+H2O���ʴ�Ϊ��2KClO3+K2SO3+H2SO4=2K2SO4+2ClO2��+H2O��
(2)��Һ©������ͨ��Һ©����ȣ����ŵ��ǵ�Һ©��Һ���Ϸ���������ƿҺ���Ϸ�ѹǿ��ȣ�ʹҺ����˳�����£�����H2SO4���ñ���ˮ��ȴ����Ϊ�˷�ֹŨ������ˮ�ų��������ȣ��Ӷ�ʹҺ��ɽ�������ֹ���ɵ�ClO2�ֽ⣬�ʴ�Ϊ����Һ©��ʹ©����������ƿ�е�ѹǿ��ͬ��ʹҺ��˳�����£����ͷ�Ӧ��ϵ���¶ȣ���ֹ�¶ȸ�ClO2�ֽ⣻
(3)ʵ�������ͨ�������Ŀ���ǽ�ClO2ȫ���ϳ��Ա�KOH��Һ���գ�ͬʱ��ϡ��ClO2����ֹŨ�ȹ������ֽ⣻�������ٹ��셼��ClO2���ܱ�������գ���������ʽ��ͣ��ʴ�Ϊ����ClO2ȫ���ϳ��Ա�KOH��Һ���գ�ͬʱ��ϡ��ClO2����ֹŨ�ȹ������ֽ⣻�������ٹ��죬ClO2���ܱ�������գ�
(4)ClO2��Cl�Ļ��ϼ�Ϊ+4�ۣ�KClO2��Cl�Ļ��ϼ�Ϊ+3�ۣ��ɴ˿�֪�÷�ӦΪ������ԭ��Ӧ���ϼ��н��ͣ��ض������ߣ���ͬʱ���п������ɵ�����ΪKClO3��.KClO4���ʴ�Ϊ��cd��
(5)KClO2���ʷֽ�ΪKClO3��KCl�����ʷ����ķ�ӦΪ��3KClO2=2KClO3+KCl��3molKClO2���ʵõ�2mol KClO3��������FeSO4��Һ��Ӧʱ��KClO2��KClO3����Fe2+��ԭΪCl-��3molKClO2��Ӧ��õ���Ϊ3mol��4=12mol��2mol KClO3��Ӧ��õ���Ϊ2mol��6=12mol��������Fe2+���ʵ�����ͬ���ʴ�Ϊ����ͬ��