��Ŀ����
����Ŀ�����������ƣ�Na2S2O5����ҽҩ����ӡȾ��ʳƷ�ȷ���Ӧ�ù㷺���ش��������⣺
��1������Na2S2O5��ͨ������NaHSO3��������Һ���ᾧ��ˮ�Ƶá�д���ù��̵Ļ�ѧ����ʽ__________��
��2�������̵����е�SO2����Na2S2O5�Ĺ���Ϊ��
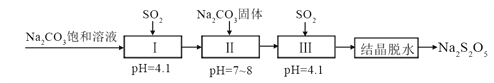
��pH=4.1ʱ������Ϊ__________��Һ��д��ѧʽ����
�������м���Na2CO3���塢���ٴγ���SO2��Ŀ����__________��
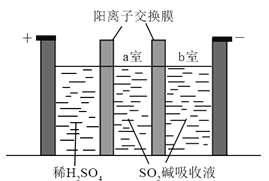
��3���Ʊ�Na2S2O5Ҳ�ɲ�������Ĥ��⼼����װ����ͼ��ʾ������SO2������Һ�к���NaHSO3��Na2SO3�������ĵ缫��ӦʽΪ_____________������__________�ҵ�NaHSO3Ũ�����ӡ���������Һ���нᾧ��ˮ���ɵõ�Na2S2O5��
��4��Na2S2O5������ʳƷ�Ŀ����������ڲⶨij���Ѿ���Na2S2O5������ʱ��ȡ50.00 mL���Ѿ���Ʒ����0.01000 mol��L1�ĵ��Һ�ζ����յ㣬����10.00 mL���ζ���Ӧ�����ӷ���ʽΪ_____________������Ʒ��Na2S2O5�IJ�����Ϊ____________g��L1����SO2�ƣ���
���𰸡� 2NaHSO3��Na2S2O5+H2O NaHSO3 �õ�NaHSO3��������Һ 2H2O��4e����4H��+O2�� a S2O52��+2I2+3H2O��2SO42��+4I��+6H�� 0.128
����������������1������ԭ���غ���д����ʽ��
��2���ٸ�����Һ�������жϲ��
��Ҫ�Ʊ����������ƣ���Ҫ�Ʊ����������ƹ�������Һ���ݴ��жϣ�
��3�����������������ŵ磬���������ӷŵ磬��������ӽ���Ĥ�����ý��
��4�������������뵥�ʵⷢ��������ԭ��Ӧ���ݴ���д����ʽ�����ݷ���ʽ�����������
��⣺��1�����������ƹ�������Һ��ˮ���ɽ��������ƣ�����ԭ���غ��֪��Ӧ�ķ���ʽΪ2NaHSO3��Na2S2O5+H2O��
��2����̼���Ʊ�����Һ����SO2�����Һ�����ԣ�˵������������ʽ�Σ�������ΪNaHSO3��
��Ҫ�Ʊ����������ƣ���Ҫ�Ʊ����������ƹ�������Һ����˹����м���̼���ƹ��塢���ٴγ�����������Ŀ���ǵõ�NaHSO3��������Һ��
��3����������ʧȥ���ӵ�������Ӧ����������ϡ���ᣬ�������ŵ磬��缫��ӦʽΪ2H2O��4e����4H��+O2��������������������ͨ�������ӽ���Ĥ����a�����������ƽ�������������ơ������������ӷŵ磬������Ũ�����������������Ʒ�Ӧ�����������ƣ����Ե���a�������������Ƶ�Ũ������
��4�����ʵ���������ԣ��ܰѽ�������������Ϊ�����ƣ���Ӧ�ķ���ʽΪS2O52��+2I2+3H2O��2SO42��+4I��+6H�������ĵ�����ʵ�����0.0001mol�����Խ��������ƵIJ���������SO2�ƣ���![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�