ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΔώ.25Γφ ±‘Ύ25 mL«β―θΜ·ΡΤ»ή“Κ÷–÷πΒΈΦ”»κ0.2 mol/L¥ΉΥα»ή“ΚΘ§ΒΈΕ®«ζœΏ»γœ¬ΆΦΥυ ΨΘ°œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «__________________

A.ΒΈΕ®÷’Βψ ±Η© ”ΕΝ ΐ‘ρ≤βΕ®ΒΡ«β―θΜ·ΡΤ≈®Ε»ΤΪΗΏ
B.BΓΔCΓΔD»ΐΒψΒΡ»ή“Κ÷–Υ°ΒΡΒγάκ≥ΧΕ»ΈΣB>C>D
C.DΒψΘ§c(CH3COO-)+ c(CH3COOH)>2c(Na+)
D.BΒψΈΣΒΈΕ®÷’Βψ
E.BΒψ ±c(CH3COO-)=c(Na+)
F. »τ”…ΧεΜΐœύΒ»ΒΡ«β―θΜ·ΡΤΚΆ¥ΉΥα»ή“ΚΜλΚœΕχ«“«ΓΚΟ≥ ÷––‘Θ§‘ρΜλΚœ«Αc(NaOH)< c(CH3COOH)
G.ΒΈΕ®«ΑΒΡ«β―θΜ·ΡΤ»ή“ΚΒΡ≈®Ε»ΈΣ0.1mol/L
Δρ.Θ®1Θ©ΈΣΝΥΦλ―ιΡ≥≤–ΝτΈο÷–Χζ‘ΣΥΊΒΡΚ§ΝΩΘ§œ»ΫΪ≤–ΝτΈο‘Λ¥ΠάμΘ§Α―Χζ‘ΣΥΊΜΙ‘≠≥…Fe2+Θ§‘Ό”ΟKMnO4±ξΉΦ»ή“Κ‘ΎΥα–‘ΧθΦΰœ¬Ϋχ––―θΜ·ΜΙ‘≠ΒΈΕ®Θ§–¥≥ωΒΈΕ®Ιΐ≥Χ÷–Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚ________________________________________KMnO4”ΠΉΑ‘Ύ___________ΒΈΕ®Ιή÷–Θ®ΧνΓΑΥα ΫΓ±ΜρΓΑΦν ΫΓ±Θ©ΒΈΕ®«Α «Ζώ“ΣΒΈΦ”÷Η ΨΦΝΘΩ___Θ®ΧνΓΑ «Γ±ΜρΓΑΖώΓ±Θ©Θ§ΒΈΕ®÷’ΒψΒΡ≈–ΕœΖΫΖ®ΘΚ_____________________________
Θ®2Θ©Ρ≥Υα–‘CuCl2»ή“Κ÷–Κ§…ΌΝΩΒΡFeCl3Θ§ΈΣ÷ΤΒΟ¥ΩΨΜCuCl2»ή“ΚΘ§“ΥΦ”»κ______________Βς÷Ν»ή“ΚpHΘΫ4Θ§ ΙFe3ΘΪΉΣΜ·ΈΣFe(OH)3≥ΝΒμΘ§¥Υ ±»ή“Κ÷–ΒΡc(Fe3ΘΪ)ΘΫ________________ΓΘ[Fe(OH)3ΒΡKsp=2.6ΓΝ10-39]
ΓΨ¥πΑΗΓΩ B E F G 5Fe2++8H++MnO4-=5Fe3++Mn2++4H2O Υα Ϋ Ζώ ΒΈΉνΚσ“ΜΒΈΥα–‘ΗΏΟΧΥαΦΊ»ή“ΚΉœ…Ϊ≤ΜΆ »ΞΘ§«“30 s≤Μ±δ…Ϊ CuO (ΜρCu(OH)2ΜρCuCO3Μρ Cu2(OH)2CO3) 2.6ΓΝ10-9mol/L
ΓΨΫβΈωΓΩΔώΓΔA.ΒΈΕ®÷’Βψ ±Η© ”ΕΝ ΐΘ§ΕΝ ΐΤΪ–ΓΘ§‘ρ≤βΕ®ΒΡ«β―θΜ·ΡΤ≈®Ε»ΤΪΒΆΘ§A¥μΈσΘΜB.ΒΈΕ®«ΑpHΘΫ13Θ§Υυ“‘«β―θΜ·ΡΤ»ή“ΚΒΡ≈®Ε» «0.1mol/LΘ§«ΓΚΟΖ¥”Π ±¥ΉΥαΡΤΥ°ΫβΘ§»ή“Κœ‘Φν–‘Θ§BΒψpHΘΫ7Θ§œ‘÷––‘Θ§ΥΒΟς¥ΉΥαΙΐΝΩΘ§“ρ¥ΥBΓΔCΓΔD»ΐΒψΒΡ»ή“Κ÷–Υ°ΒΡΒγάκ≥ΧΕ»ΈΣB>C>DΘ§B’ΐ»ΖΘΜC.DΒψΒΟΒΫΒΡ»ή“Κ «Β»≈®Ε»ΒΡ¥ΉΥαΡΤΚΆ¥ΉΥαΒΡΜλΚœ»ή“ΚΘ§ΗυΨίΈοΝœ ΊΚψΩ…÷Σc(CH3COO-)+c(CH3COOH)ΘΫ2c(Na+)Θ§C¥μΈσΘΜD.BΒψœ‘÷––‘Θ§≤Μ «ΒΈΕ®÷’ΒψΘ§D¥μΈσΘΜE.BΒψ ±œ‘÷––‘Θ§ΗυΨίΒγΚ… ΊΚψΩ…÷Σc(CH3COO-)=c(Na+)Θ§E’ΐ»ΖΘΜF. »τ”…ΧεΜΐœύΒ»ΒΡ«β―θΜ·ΡΤΚΆ¥ΉΥα»ή“ΚΜλΚœΕχ«“«ΓΚΟ≥ ÷––‘Θ§‘ρ¥ΉΥα“ΜΕ®ΙΐΝΩΘ§Υυ“‘ΜλΚœ«Αc(NaOH)<c(CH3COOH)Θ§F’ΐ»ΖΘΜG.ΒΈΕ®«ΑΒΡ«β―θΜ·ΡΤ»ή“ΚΒΡ≈®Ε»ΈΣ0.1mol/LΘ§G’ΐ»ΖΘ§¥πΑΗ―ΓBEFGΘΜ
Δρ.Θ®1Θ©ΒΈΕ®Ιΐ≥Χ÷–―«ΧζάκΉ”±Μ―θΜ·Θ§Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ5Fe2++8H++MnO4-ΘΫ5Fe3++Mn2++4H2OΘΜKMnO4ΨΏ”–«Ω―θΜ·–‘Θ§«“ «ΥαΜ·ΒΡΘ§Υυ“‘”ΠΉΑ‘ΎΥα ΫΒΈΕ®Ιή÷–ΘΜΥα–‘ΗΏΟΧΥαΦΊ»ή“Κœ‘Ήœ…ΪΘ§Υυ“‘ΒΈΕ®«Α≤Μ–η“ΣΒΈΦ”÷Η ΨΦΝΘ§ΒΈΕ®÷’ΒψΒΡ≈–ΕœΖΫΖ®ΈΣΘΚΉνΚσ“ΜΒΈΥα–‘ΗΏΟΧΥαΦΊ»ή“ΚΉœ…Ϊ≤ΜΆ »ΞΘ§«“30 s≤Μ±δ…ΪΘΜΘ®2Θ©”…”Ύ≤ΜΡή“ΐ»κ–¬‘”÷ Θ§‘ρ”ΠΗΟΦ”»κCuOΜρCu(OH)2ΜρCuCO3ΜρCu2(OH)2CO3)Βς÷Ν»ή“ΚpHΘΫ4ΓΘ»ή“Κ÷–«β―θΗυΒΡ≈®Ε» «10Θ≠10mol/LΘ§Υυ“‘ΗυΨί«β―θΜ·ΧζΒΡ»ήΕ»Μΐ≥Θ ΐΩ…÷Σ»ή“Κ÷–c(Fe3ΘΪ)ΘΫ mol/LΓΘ
mol/LΓΘ

 Ά§≤ΫΝΖœΑ«ΩΜ·ΆΊ’ΙœΒΝ–¥πΑΗ
Ά§≤ΫΝΖœΑ«ΩΜ·ΆΊ’ΙœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΡ≥―ßœΑ–ΓΉιΕ‘Ρ≥ΤœΧ―ΨΤ÷–SO2ΒΡΚ§ΝΩΫχ––Φλ≤βΘ§Α¥œ¬ΆΦΝ§Ϋ”ΚΟ“«ΤςΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ

Δώ.―υΤΖ’τΝσ
»Γ20.00mL―υΤΖΚΆ250 mLΥ°÷Ο”Ύ“«ΤςA÷–Θ§“«ΤςB «»ΞΒτ»ϊΉ”ΒΡΒβΝΩΤΩΘ§œρΤδ÷–ΉΑ»Υ25mL ““Υα«Π»ή“ΚΘ§»ΜΚσœρ“«ΤςA÷–Φ”»κ10 mL―ΈΥαΘ§ΝΔΦ¥Η«»ϊ’τΝσΘΜΒ±ΒβΝΩΤΩ÷–ΒΡ“ΚΧε‘Φ200 mL ±Θ§ΫΪ≤ε»κΒβΝΩΤΩ“ΚΟφœ¬ΒΡΒΦΙήœρ…œΧαΤπ≤ΔΫΪΒΦΙή÷Ο”Ύ“ΚΟφ…œΖΫ1cm ¥ΠΘ§‘Ό’τΝσ2minΉσ”“ΘΜΉνΚσ”Ο…ΌΝΩ’τΝσΥ°≥εœ¥ΒβΝΩΤΩ÷–ΒΡΒΦΙήΘ§≤ΔΫΪ≥εœ¥“Κ≤Δ»κΒβΝΩΤΩ÷–ΓΘΆ§ ±ΉωΩ’ΑΉ Β―ι(Φ¥≤ΜΦ”―υΤΖ«ιΩωœ¬Α¥Ά§Ζ®≤ΌΉςΘ§Ζά÷Ι Β―ιΈσ≤ν)ΓΘ
“―÷ΣΘΚSO2+H2O+Pb(CH3COO)2=PbSO3Γΐ+2CH3COOHΓΘ
(1)“«ΤςA ΒΡΟϊ≥Τ «_________________ ΓΘ
(2) Β―ι÷– Ι”ΟΒΡΒβΝΩΤΩΉν “ΥΒΡΙφΗώ «_________ (Χν±ξΚ≈)ΓΘ
a.100 mL b.250 mL c.500 mL d.1000 mL
(3)»γΙϊ Β―ιΉνΚσΈ¥”Ο…ΌΝΩ’τΝσΥ°≥εœ¥ΒβΝΩΤΩ÷–ΒΡΒΦΙήΘ§ΜαΒΦ÷¬≤βΕ®ΫαΙϊ__________(ΧνΓΑΤΪΗΏΓ±ΓΑ ΤΪΒΆΓ±ΜρΓΑ≤Μ”ΑœλΓ±)ΓΘ
Δρ.ΒΈΕ®≤ΌΉς
œρ»Γœ¬ΒΡΒβΝΩΤΩ÷–Φ”»κ10 mL―ΈΥαΚΆ1mLΒμΖέ»ή“ΚΘ§“Γ‘»Κσ”Ο0.01molΓΛL-1ΒΡΒβ±ξΉΦ»ή“ΚΒΈΕ®Θ§Φ«¬ΦœϊΚΡΒΡΒβ±ξΉΦ»ή“ΚΒΡΧεΜΐΈΣV( ΒΞΈΜΈΣmL)ΓΘ
(4)ΒΈΕ®÷’ΒψΒΡœ÷œσ «_____________________Θ§ΗΟΒΈΕ®Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «______________ΓΘ
Δσ.ΫαΙϊΖ÷Έω
(5)ΦΉΉι4 ¥Έ Β―ι≤βΒΟVΖ÷±πΈΣ
Β―ι | 1 | 2 | 3 | 4 |
V/mL | 9.98 | 12.00 | 10.02 | 10.00 |
Ψί¥ΥΩ…ΦΤΥψ¥ΥΤœΧ―ΨΤ÷–SO2ΒΡΚ§ΝΩΈΣ_______mgΓΛL-1
““Ήι»œΈΣΦΉΉι Β―ι≤Μ―œΫςΘ§Ω…ΡήΜα”–Έσ≤νΘ§“ρΈΣΦΉΉιΈ¥Ήω_____________________ΓΘ
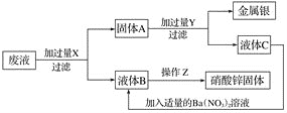
ΓΨΧβΡΩΓΩœ¬Ν–ΗςΉιΈο÷ ÷–Θ§X±μ ΨΡ≥ΈοΘ§Y±μ ΨXΈο÷ ÷–Κ§”–ΒΡ…ΌΝΩ‘”Θ§Z±μ Ψ“Σ≥ΐ»Ξ‘”÷ Φ”»κΒΡ ‘ΦΝΘ§Τδ÷–’ΐ»ΖΒΡΉι±π «Θ® Θ©
X | Y | Z | |
A | FeCl2 »ή“Κ | FeCl3 | KSCN »ή“Κ |
B | H2 | SO2 | NaOH »ή“Κ |
C | Fe2Θ®SO4Θ©3»ή“Κ | FeSO4 | Cl2 |
D | SiO2 | Fe2O3 | NaOH »ή“Κ |
A.A
B.B
C.C
D.D