��Ŀ����
��12�֣��ڻ�ѧ��ѧ�о��У����ʷ�����ѧ��Ӧ�ķ�Ӧ�ȿ�ͨ��ʵ��ⶨ��Ҳ��ͨ����ѧ����ķ�ʽ��ӵػ�á�
��ʵ�鷽���ⷴӦ��Ӧ��
���к��Ȳⶨ
ʵ��������Ҫʹ�õIJ����������ձ�����Ͳ���Ҫ �� ��
Ϊ�˼���ʵ����ʵ������н�NaOH��Һ ��ѡ�һ�Ρ��ֶ�Ρ�������ʢ�������С�ձ��У���Һ��Ϻ�ȷ��ȡ�����Һ�� ����Ϊ��ֹ�¶ȡ�
��ʵ��ⶨ��һ���¶��£�0.2 molCH4(g)������H2O(g)��ȫ��Ӧ����CO2(g)��H2(g)����33 kJ���������÷�Ӧ���Ȼ�ѧ����ʽ ��
��ͨ����ѧ�����ӻ��
����֪��1mol��H��H����I��I��H��I���ֱ���Ҫ���յ�����Ϊ436kJ��153kJ��299kJ��
��ӦH2(g)��I2(g)��2HI(g)�ķ�Ӧ�ȡ�H�� kJ��mol��1
�ڹ�ҵ�����״��ij��÷����ǣ�CO(g)+2H2(g)��CH3OH(g) ��H����90.8 kJ��mol��1��
��֪��2H2(g)+ O2(g) �� 2H2O (l) ��H����571.6 kJ��mol��1
H2(g)+ O2(g) �� H2O(g) ��H����241.8 kJ��mol��1
O2(g) �� H2O(g) ��H����241.8 kJ��mol��1
����������Ӧȷ����H2ȼ����Ϊ kJ��mol��1��
CH3OH(g)��O2(g) �� CO(g)��2H2O(g) ��H�� kJ��mol��1
��ʵ�鷽���ⷴӦ��Ӧ��
���к��Ȳⶨ
ʵ��������Ҫʹ�õIJ����������ձ�����Ͳ���Ҫ �� ��
Ϊ�˼���ʵ����ʵ������н�NaOH��Һ ��ѡ�һ�Ρ��ֶ�Ρ�������ʢ�������С�ձ��У���Һ��Ϻ�ȷ��ȡ�����Һ�� ����Ϊ��ֹ�¶ȡ�
��ʵ��ⶨ��һ���¶��£�0.2 molCH4(g)������H2O(g)��ȫ��Ӧ����CO2(g)��H2(g)����33 kJ���������÷�Ӧ���Ȼ�ѧ����ʽ ��
��ͨ����ѧ�����ӻ��
����֪��1mol��H��H����I��I��H��I���ֱ���Ҫ���յ�����Ϊ436kJ��153kJ��299kJ��
��ӦH2(g)��I2(g)��2HI(g)�ķ�Ӧ�ȡ�H�� kJ��mol��1
�ڹ�ҵ�����״��ij��÷����ǣ�CO(g)+2H2(g)��CH3OH(g) ��H����90.8 kJ��mol��1��
��֪��2H2(g)+ O2(g) �� 2H2O (l) ��H����571.6 kJ��mol��1
H2(g)+
 O2(g) �� H2O(g) ��H����241.8 kJ��mol��1
O2(g) �� H2O(g) ��H����241.8 kJ��mol��1����������Ӧȷ����H2ȼ����Ϊ kJ��mol��1��
CH3OH(g)��O2(g) �� CO(g)��2H2O(g) ��H�� kJ��mol��1
��12�֣�
���¶ȼ� ���β�������� һ�� ����¶� (��1��)
CH4(g)��2H2O(g) ��CO2(g)��4H2(g) ��H����165.0 kJ��mol��1
�� ��9 285.8 ��392.8 ��ÿ��2�֣�
���¶ȼ� ���β�������� һ�� ����¶� (��1��)
CH4(g)��2H2O(g) ��CO2(g)��4H2(g) ��H����165.0 kJ��mol��1
�� ��9 285.8 ��392.8 ��ÿ��2�֣�
������������к��Ȳⶨ
ʵ��������Ҫʹ�õIJ����������ձ�����Ͳ���Ҫ�¶ȼ� ���β����������
Ϊ�˼���ʵ����ʵ������н�NaOH��Һһ�ε���ʢ�������С�ձ��У���Һ��Ϻ�ȷ��ȡ�����Һ������¶ȣ���Ϊ��ֹ�¶ȡ�
��ʵ��ⶨ��һ���¶��£�0.2 molCH4(g)������H2O(g)��ȫ��Ӧ����CO2(g)��H2(g)����33 kJ���������÷�Ӧ���Ȼ�ѧ����ʽCH4(g)��2H2O(g) ��CO2(g)��4H2(g) ��H����165.0 kJ��mol��1��
��ͨ����ѧ�����ӻ��
����֪��1mol��H��H����I��I��H��I���ֱ���Ҫ���յ�����Ϊ436kJ��153kJ��299kJ��
��ӦH2(g)��I2(g)��2HI(g)�ķ�Ӧ�ȡ�H��436kJ+153kJ-2��299kJ=��9 kJ��mol��1
�ڹ�ҵ�����״��ij��÷����ǣ�CO(g)+2H2(g)��CH3OH(g) ��H1����90.8 kJ��mol��1��
��֪��2H2(g)+ O2(g) �� 2H2O (l) ��H2����571.6 kJ��mol��1
H2(g)+
 O2(g) �� H2O(g) ��H3����241.8 kJ��mol��1
O2(g) �� H2O(g) ��H3����241.8 kJ��mol��1����������Ӧȷ����H2ȼ����Ϊ285.8kJ��mol��1��
CH3OH(g)��O2(g) �� CO(g)��2H2O(g) ��H��-��H1-��H2+2��H3=��392.8kJ��mol��1��
����������Ҫ��ѧ����Ϥ�к��ȵIJⶨʵ�飺����ʵ����̣�ʵ��������ʵ�����ݷ����ȵȣ���˹����Ҳ�dz�������Ŀ��ʹ�øö���Ҫע�⣺
1����˹����ֻ�����ڵ��µ�ѹ����µ��ݹ��̣�������Ӧ���¶�Ӧ��ͬ��
2����ЧӦ����뷴Ӧ�ĸ����ʵı��ԡ��ۼ�״̬����ɷ�Ӧ��������������Ӧ���еķ�ʽ���¶ȡ�ѹ�������ؾ��йأ����Ҫ���漰�ĸ�����Ӧʽ�������ϸ��������Ȼ�ѧ����ʽ��
3��������Ӧ���������������
4�������漰��ͬһ����Ӧ������ͬ�ľۼ�״̬��
5����ѧ��Ӧ�ķ�Ӧ��(��H)ֻ�뷴Ӧ��ϵ��ʼ̬����̬�й�,���뷴Ӧ;���ء�

��ϰ��ϵ�д�
 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�
�����Ŀ
 ����Һ
����Һ
 ��Һ
��Һ
 ZnO
ZnO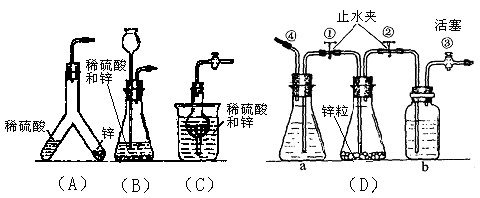
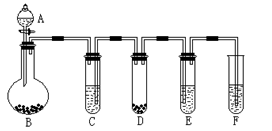
 װ��D�У�
װ��D�У�  װ��F�У�
װ��F�У�