��Ŀ����
����Ŀ��ʵ����������1mol/L NaOH��Һ240ml����ش�
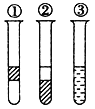
��1�����ȡNaOH����____________g��������Ϊ23.1 g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ�����ڸ�����ѡȡ����������С___________(����ĸ)��������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ��____________(����ĸ)��
����������
a | b | c | d | e | |
�����С/g | 100 | 50 | 20 | 10 | 5 |

��2��ѡ�õ���Ҫ�����������ձ������������_____________________________��
��3�����в�����˳���ǣ�����ű�ʾ��_________________��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������ƽȷ��ȡ�����NaOH����������������ˮ���ò���������������ʹ�����ܽ�
C��������ȴ��NaOH��Һ�ز�����ע������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
��4����������������Ƶ�NaOH��ҺŨ���к�Ӱ�죿������ƫ������ƫС������Ӱ������д��
������ƿ������ϴ�Ӻ������������ˮ________________
�����ȵ���Һֱ������������ƿ________________
������ʱ����������ƿ�Ŀ̶���________________
��ת����Һʱ�����������¶�������ƿ�Ŀ̶�������________________
���𰸡� 10.0 cd h 250ml����ƿ ��ͷ�ι� BCAFED ��Ӱ�� ƫ�� ƫС ƫС
��������(1)����240mL1molL-1NaOH��Һ��Ҫ�������Ƶ�����Ϊ0.25L��1mol/L��40g/mol=10.0g��
�������ƾ��и�ʴ�����׳��⣬Ӧ�����ձ��ڳ��������������������ձ�������Ϊ10.0g+23.1g=33.1g����Ӧѡ��20g��10g�����룬��ѡ��cd���ɱ������߿�֪����С������Ϊ5g������������̶�Ϊ5g����С��5g����������Ӧ��3.1g��λ�ã���ѡ��h��
(2)�������һ�����ʵ���Ũ�ȵ���Һ����������裬��Ҫ�õ���������������ƽ��ҩ�ס��ձ���������������ƿ����ͷ�ιܣ�����250mL1.0mol/L��NaOH��Һ��Ӧѡ��2500mL����ƿ����ȱ�ٵIJ���������250ml����ƿ�ͽ�ͷ�ιܣ�
(3)����һ�����ʵ���Ũ�ȵ���Һһ����������ǣ����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ�������˳��ΪBCAFED��
(4)��������������Ƶ�NaOH��ҺŨ���к�Ӱ�죿(����ƫ������ƫС������Ӱ������д)
��������ƿδ���T����������Һ������ҺŨ����Ӱ�죬��ΪֻҪ����ʱ��ȷ������ˮ��ԭ�����еĻ��Ǻ�������ģ���Ũ����Ӱ�죻
�ڽ��ȵ���Һֱ������������ƿ�����ݺ���Һ��ȴҺ����½�������Һ�����ƫС����ҺŨ��ƫ��
�۶���ʱ����Һ�棬������Һ���ƫ����Ũ�Ȼ�ƫС��
��ת����Һʱ���������¶˿�������ƿ�̶������ϣ���̶����ϸ��ŵ���Һ��������Һ���ƫ��Ũ��ƫС��