��Ŀ����
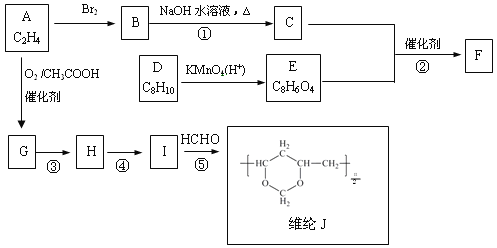
����Ŀ���ȼ��ⱥ��ʳ��ˮ��ȡNaOH�Ĺ�������ʾ��ͼ���£�
������ͼ�����������գ�
(1)�ڵ������У����Դ���������ĵ缫����������Ӧ�ĵ缫��ӦʽΪ_________________�����Դ���������ĵ缫��������ҺpH________(ѡ����䡱�������ߡ����½���)��
(2)��ҵʳ���к�Ca2����Mg2�������ʣ����ƹ����г�ȥ��Щ����ʱ������Ӧ�����ӷ���ʽΪ__________________��__________________��
(3)���������SO42���������ߣ��������ӱ��Լ���ȥSO42�����ñ��Լ�������________(��д��ĸ��ţ���ͬ)��
A��Ba(OH)2 B��Ba(NO3)2 C��BaCl2
(4)Ϊ��Ч��ȥCa2����Mg2����SO42���������Լ��ĺ���˳��Ϊ________��
A���ȼ�NaOH�����Na2CO3���ټӱ��Լ�
B���ȼ�NaOH����ӱ��Լ����ټ�Na2CO3
C���ȼӱ��Լ������NaOH���ټ�Na2CO3
(5)���ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ��________����ȴ��________(��д��������)��ȥNaCl��
(6)�ø�Ĥ�����ʳ��ˮʱ�����۷ָ�Ϊ������������������ֹCl2��NaOH��Ӧ��������Ĥ��������ʳ��ˮʱ��Cl2��NaOH��ֽӴ����õ��IJ������NaClO��H2������÷�Ӧ��Ӧ�Ļ�ѧ����ʽΪ___________________��
���𰸡�(1)2Cl����2e��==Cl2�� ����
(2)Ca2����CO32��==CaCO3��
Mg2����2OH��==Mg(OH)2��
(3)AC (4)BC (5)���� ����
(6)NaCl��H2O![]() NaClO��H2��
NaClO��H2��
(��2NaCl��2H2O![]() H2����Cl2����2NaOH��Cl2��2NaOH==NaCl��NaClO��H2O)
H2����Cl2����2NaOH��Cl2��2NaOH==NaCl��NaClO��H2O)
��������(1)��ⱥ��ʳ��ˮ�����Դ���������ĵ缫Ϊ�������缫��Ӧʽ��2Cl����2e��==Cl2�������Դ���������ĵ缫Ϊ�������缫��Ӧʽ��2H����2e��==H2��������H��������OH����������Һ��pH���ߡ�
(2)��ȥCa2����Ca2����CO32��==CaCO3������ȥMg2����Mg2����2OH��==Mg(OH)2��������Mg(OH)2���ܽ�����С��MgCO3������Mg2����CO32����OH�����ʱ��һ��������Mg(OH)2������
(3)��ȥSO42����Ӧѡ��Ba2�����������Ba(NO3)2����Һ�л������µ����ʣ�����Ba(OH)2��BaCl2���������µ����ʣ�ѡA��C��
(4)��ȥ����ʱ����Ba2����OH�������Ⱥ�֮�֣���Na2CO3һ��Ҫ�������룬��ΪCO32����Ҫ��ȥ�����Ba2�������˳�������ΪNaCl���ᴿ��ѡB��C��
(5)����ʵ�����Ƿ���NaOH��NaCl������NaCl�ܽ��С�����NaCl�����������������������������ȴ���ᾧ�������˵�NaCl���壬��Һ��һ�����Ƶ�NaOH��
(6)��2NaCl��2H2O![]() 2NaOH��H2����Cl2����Cl2��2NaOH==NaCl��NaClO��H2O�ϲ������ɵó��𰸡�
2NaOH��H2����Cl2����Cl2��2NaOH==NaCl��NaClO��H2O�ϲ������ɵó��𰸡�

����Ŀ����֪�����������з�Ӧ��2Cu��=Cu2����Cu��������ԭ����ͭʵ�����ڷ�Ӧ�¶Ȳ�ͬ�����ܲ���Cu��Cu2O�����߶��Ǻ�ɫ���塣ijͬѧ��ij��������ԭ����ͭ�ĺ�ɫ���������������ʵ�飬ʵ�������ʵ�������б����£�
���� �Լ� | ϡ���� | Ũ���� ������ | ϡ���� | Ũ���� |
ʵ�� ���� | ��ɫ���� ����Ӧ | ��ɫ���� | ��ɫ���� ��ɫ��Һ | ����ɫ���� ��ɫ��Һ |
�ɴ��Ƴ�����������ԭ����ͭʵ��IJ���(����)
A. ��Cu B. ��Cu2O C. һ����Cu��һ����Cu2O D. һ����Cu2O��������Cu