��Ŀ����
10������˵����ȷ���ǣ�������| A�� | �����£�pH=3��HX��һԪ�ᣩ��Һ��pH=11��YOH��һԪ���Һ�������ϣ�������Һ��pHһ�����ڻ����7 | |
| B�� | ����ͬ�¶��£�Ũ�Ⱦ�Ϊ0.1 mol•L-1�ģ�NH4��2Fe��SO4��2�ͣ�NH4��2SO4��Һ�У�c��NH4+����ͬ | |
| C�� | ��pH��7��CH3COOH��CH3COONa�Ļ��Һ�У�c��Na+����c��CH3COO-����c��H+����c��OH-�� | |
| D�� | 0.4 mol•L-1������0.1 mol•L-1NaAlO2��Һ�������ϣ�������Һ�У�c��Cl-����c��Na+����c��Al3+����c��H+����c��OH-�� |
���� A����HXΪ���ᣬYOHΪǿ�����������Һ��pH��7��
B����NH4��2Fe��SO4��2�е�Fe2+��ˮ���������NH4+��ˮ�⣻
C��pH��7����c��H+����c��OH-�������ݵ���غ��֪��c��CH3COO-����c��Na+����
D����Ӧ����ҺΪ��Ũ�ȵ�AlCl3��NaCl�Ļ����Һ�������Ӳ���ˮ�⣬��Һ��ʾ���ԣ���c��Na+����c��Al3+����c��H+����c��OH-����
��� �⣺A����HXΪǿ�ᣬYOHΪǿ���pH=7����HXΪ���ᣬYOHΪǿ���pH��7����HXΪǿ�ᣬYOHΪ�����pH��7����A����
B������Fe2+��ˮ���������NH4+��ˮ�⣬�ʣ�NH4��2Fe��SO4��2��Һ�е�c��NH4+���ȣ�NH4��2SO4�еĴ�B����
C�����ݹ�ϵʽ��֪c��Na+��+c��H+����c��CH3COO-��+c��OH-�������˹�ϵʽ�����ϵ���غ㣬��ȷ������Ũ�ȴ�СΪ��c��CH3COO-����c��Na+����c��H+����c��OH-������C����
D.0.4mol•L-1������0.1 mol•L-1NaAlO2��Һ�������ϣ�������ҺΪ��Ũ�ȵ�AlCl3��NaCl�Ļ����Һ�������Ӳ���ˮ�⣬��Һ��ʾ���ԣ���c��Na+����c��Al3+����c��H+����c��OH-������Һ������Ũ�ȴ�СΪ��c��Cl-����c��Na+����c��Al3+����c��H+����c��OH-������D��ȷ��
��ѡD��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ�ε�ˮ��ԭ������Ӱ��Ϊ���ؼ���ע�����յ���غ㡢�����غ�ĺ��弰Ӧ�÷���������������ѧ���ķ������������������Ӧ�û���֪ʶ��������

| A�� | 100ml 5mol/L KCl | B�� | 250ml 5mol/L MgCl2 | ||
| C�� | 100ml 1mol/L CaCl2 | D�� | 250ml 1mol/L NaCl |
| A�� | 0.1 mol•L-1CH3COONa��Һ��0.1 mol•L-1HCl��Һ�������ϣ�c��Na+��=c��Cl-����c��H+��=c��OH-�� | |
| B�� | 0.1 mol•L-1NH4Cl��Һ��0.1 mol•L-1��ˮ�������ϣ�pH��7����c��NH3•H2O����c��NH${\;}_{4}^{+}$����c��Cl-����c��OH-�� | |
| C�� | 0.1 mol•L-1Na2CO3��Һ��0.1 mol•L-1NaHCO3��Һ�������ϣ�$\frac{2}{3}$c��Na+��=c��CO${\;}_{3}^{2-}$��+c��HCO${\;}_{3}^{-}$��+c��H2CO3�� | |
| D�� | 0.1 mol•L-1Na2C2O4��Һ��0.1 mol•L-1HCl��Һ�������ϣ�H2C2O4Ϊ��Ԫ���ᣩ��2c��C2O${\;}_{4}^{2-}$��+c��HC2O${\;}_{4}^{-}$��+c��OH-��=c��Na+��+c��H+�� |
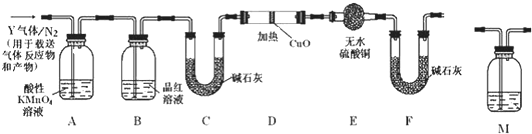
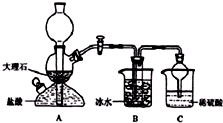 ��ʵ�����У�������̼�����ơ��Ȼ��ơ��Ȼ�淋������ܽ�ȵIJ��죬ͨ������ʳ��ˮ�����Ͷ�����̼��Ӧ�����̼�����ƾ��壬��Ӧԭ���������»�ѧ����ʽ��ʾ��NH3+CO2+NaCl+H2O�TNH4Cl+NaHCO3�������ݴ�ԭ�������Ƶ�̼�����ƾ��壬ijУѧ���������ͼʵ��װ�ã�����Bװ���е��Թ��������а����Ȼ��Ƶ���Һ���Ҷ��߾��Ѵﵽ���ͣ�
��ʵ�����У�������̼�����ơ��Ȼ��ơ��Ȼ�淋������ܽ�ȵIJ��죬ͨ������ʳ��ˮ�����Ͷ�����̼��Ӧ�����̼�����ƾ��壬��Ӧԭ���������»�ѧ����ʽ��ʾ��NH3+CO2+NaCl+H2O�TNH4Cl+NaHCO3�������ݴ�ԭ�������Ƶ�̼�����ƾ��壬ijУѧ���������ͼʵ��װ�ã�����Bװ���е��Թ��������а����Ȼ��Ƶ���Һ���Ҷ��߾��Ѵﵽ���ͣ���1��Aװ������������Ӧ�����ӷ���ʽΪ��CaCO3+2H+�TCa2++CO2��+H2O��Cװ����ϡ���������Ϊ�����մ�Bװ���е��Թ����ݳ��İ��������ٶԻ�������Ⱦ��
��2���±������г�������������ڲ�ͬ�¶��µ��ܽ�����ݣ�g/100gˮ��
| 0�� | 10�� | 20�� | 30�� | 40�� | 50�� | |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 |
| NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 |
| NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 |
��3����Уѧ���ڼ�������װ�������Ժ����ʵ�飬���û�еõ�̼�����ƾ��壬ָ����ʦָ��Ӧ��Aװ��B֮�䣨��д��ĸ������һ��ʢ�б���NaHCO3��Һ ��ϴ��װ�ã��������dz�ȥCO2�л��е�HCl���壮
��4�����øĽ����װ�ý���ʵ�飬��B�е��Թ��������˾��壬����Ҫ�IJ�����õ���һ�ִ����ľ��壮��ͨ����ʵ���жϸþ�����̼�����ƾ��������̼����茶��壬��������������ʵ�����ȡ�������������Թ��У��ھƾ����ϼ���ʹ���ַ�Ӧ���а�ɫ����ʣ�࣬��
���岻��NH4HCO3��
��5������Уѧ������ʵ��ʱ�����ñ���ʳ��ˮ�к�NaCl������Ϊ5.85g��ʵ���õ������NaHCO3���������Ϊ5.88g����NaHCO3�IJ���Ϊ70%��
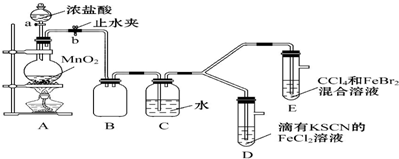
��1��������巢��װ��A�������ԵIJ����ǹر�ֹˮ��b������a�����Һ©����עˮ����ˮ����˳�����£������������ã�
��2��ʵ��������£�
| ʵ����� | ʵ������ | ���� |
| ����a����Բ����ƿ�е�������Ũ���Ȼ��رջ���a����ȼ�ƾ��� | Dװ���У���Һ���ɫ��Eװ���У�ˮ����Һ���ɫ����CCl4�������Ա仯 | Cl2��Br2��Fe3+��������ǿ������˳��ΪCl2��Br2��Fe3+ |
��Ϊ��̽����Һ����ɫ������ȥ����ԭ��������ʵ�飮
ȡD����Һ���Թ��У�����NaOH��Һ���к��ɫ�������ɣ�����Һ��һ������Fe3+��ȡD����Һ���Թ��У����������KSCN��Һ�����յõ���ɫ��Һ��ʵ��֤������ɫ������ȥ����ԭ���ǹ���������SCN-��Ӧ2SCN-+Cl2=2Cl-+��SCN��2��ʹSCN-Ũ�ȼ�С����ʹFe3++3SCN-?Fe��SCN��3ƽ�����淴Ӧ�����ƶ�����ɫ��
�ڲ²�SCN-���ܱ�Cl2�����ˣ��ֽ����������о���
������ʾ��
SCN-�ĵ���ʽΪ
 ����ΪSCN-��̼Ԫ��û�б�������������SCN-��̼Ԫ�������̬+4�ۣ�ȡD����Һ���Թ��У������������ữ��BaCl2��Һ��������ɫ�������ɴ�֤��SCN-�б�������Ԫ������Ԫ�أ�ͨ��ʵ��֤����SCN-�е�Ԫ��ת��ΪNO3-����SCN-��Cl2��Ӧ����1mol CO2����ת�Ƶ��ӵ����ʵ�����16mol��
����ΪSCN-��̼Ԫ��û�б�������������SCN-��̼Ԫ�������̬+4�ۣ�ȡD����Һ���Թ��У������������ữ��BaCl2��Һ��������ɫ�������ɴ�֤��SCN-�б�������Ԫ������Ԫ�أ�ͨ��ʵ��֤����SCN-�е�Ԫ��ת��ΪNO3-����SCN-��Cl2��Ӧ����1mol CO2����ת�Ƶ��ӵ����ʵ�����16mol����4������ʵ��װ�ô���һ�����Բ��㣬��������ķ����ڻ�����Ӧ�ĸĽ�װ�ã�

| A�� | ������������Ʒ�Ӧ | B�� | �Ȼ�������ˮ | ||
| C�� | �廯��Ͱ���Ӧ | D�� | п��ϡ���ᷴӦ |
����ȷ���ǣ�������
| A�� | �������������µ�״̬�ǹ��壬��ɫ�ȵ���������������Ȼ�̼ | |
| B�� | ��ԭ�ӵõ��������ȵ�ǿ | |
| C�� | AgAt����ɫ��AgI �HAt���ȶ��Ա�HI�� | |
| D�� | ��������±������������������������±���е�ǿ��ԭ�� |
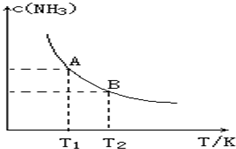 ��һ�������£���1.00molN2��g����3.00molH2��g�������һ��10.0L�ܱ������У��ڲ�ͬ�¶��´ﵽƽ��ʱNH3��g����ƽ��Ũ����ͼ��ʾ�������¶�ΪT1ʱƽ���������а������������Ϊ25.0%��
��һ�������£���1.00molN2��g����3.00molH2��g�������һ��10.0L�ܱ������У��ڲ�ͬ�¶��´ﵽƽ��ʱNH3��g����ƽ��Ũ����ͼ��ʾ�������¶�ΪT1ʱƽ���������а������������Ϊ25.0%��