��Ŀ����
����Ŀ���밴Ҫ����գ�
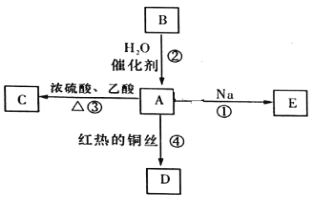
��1��д��![]() �к��������ŵ�����_______________��
�к��������ŵ�����_______________��
��2��![]() ����__________����
����__________����
��3��CH2=CH��CH3�ڴ����������������ɾۺ���ķ�Ӧ����ʽΪ________��
��4��д��ʵ��������Ȳ�Ļ�ѧ��Ӧ����ʽ_________________��
��5���ٳ�ȡ3.4gij�л�������A����ȫȼ�պ�����1.8g H2O��8.8g CO2����֪���л��������������������ܶ�Ϊ68������л���ķ���ʽΪ_________________��
�ڸ��л���A�ĺ˴Ź������ͺ���������£�
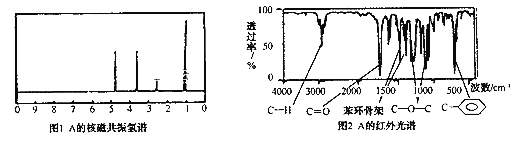
���Ʋ��л���A�Ľṹ��ʽΪ_________________________��
����A����ͬ���л����ͬ���칹�����A����_________�֡�
���𰸡�ȩ�� �� nCH3CH=CH2![]() CaC2+2H2O��Ca(OH)2+CH��CH�� C8H8O2
CaC2+2H2O��Ca(OH)2+CH��CH�� C8H8O2 ![]() 6
6
��������
��1��![]() �к��������ŵ�����ȩ����
�к��������ŵ�����ȩ����
��2��![]() ��������������������
��������������������
��3��CH2=CH��CH3����̼̼˫�����ڴ������������·����Ӿ۷�Ӧ���ɾ۱�ϩ����Ӧ����ʽΪnCH3CH=CH2![]() ��
��
��4��ʵ�����õ�ʯ��ˮ��Ӧ��ȡ��Ȳ����ѧ��Ӧ����ʽ��CaC2+2H2O��Ca(OH)2+CH��CH����
��5���٣�1��n��CO2��=![]() 0.2mol����3.4g�л����У�n��C��=n��CO2��=0.2mol��m��C��=0.2mol��12g/mol=2.4g�� n��H2O��=
0.2mol����3.4g�л����У�n��C��=n��CO2��=0.2mol��m��C��=0.2mol��12g/mol=2.4g�� n��H2O��=![]() 0.1mol����3.4g�л����У�n��H��=2n��H2O��=0.2mol��m��H��=0.2mol��1g/mol=0.2g����2.4g+0.2g����3.4g�������л����л�Ӧ��OԪ�أ���m��O��=3.4g-2.4g-0.2g=0.8g��n��O��=
0.1mol����3.4g�л����У�n��H��=2n��H2O��=0.2mol��m��H��=0.2mol��1g/mol=0.2g����2.4g+0.2g����3.4g�������л����л�Ӧ��OԪ�أ���m��O��=3.4g-2.4g-0.2g=0.8g��n��O��=![]() 0.05mol�����л����У�n��C����n��H����n��O��=0.2mol��0.2mol��0.05mol=4��4��1�����Ը��л�������ʽΪC4H4O����֪���л��������������������ܶ�Ϊ68������л�����Է���������136,���Է���ʽΪΪC8H8O2��
0.05mol�����л����У�n��C����n��H����n��O��=0.2mol��0.2mol��0.05mol=4��4��1�����Ը��л�������ʽΪC4H4O����֪���л��������������������ܶ�Ϊ68������л�����Է���������136,���Է���ʽΪΪC8H8O2��
�ڸ���A�ĺ˴Ź������ף�A��4�ֵ�Ч�⣬������Ϊ3��2��2��1������A�ĺ�����ף�A����![]() ��
��![]() ��
��![]() �ṹ������A�Ľṹ��ʽΪ
�ṹ������A�Ľṹ��ʽΪ![]() ��
��
����A����ͬ���л����ͬ���칹����![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ������
������![]() ����6�֡�
����6�֡�

 ��У����ϵ�д�
��У����ϵ�д�