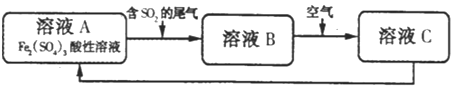
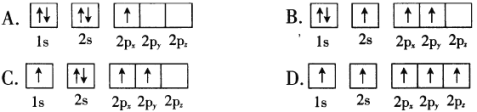
��Ŀ����
����Ŀ��I.��ͼ�Ǹ�����ij��ͯ��Ԫ����챨�浥�IJ������ݣ�
������ijҽ�ƻ����ٴ����������浥 | ||||
������Ŀ | ����� | ��λ | ����ֵ�ο���Χ | |
1 | �Zn�� | 109.62 | ��mol/L | 66-120 |
2 | ����Fe�� | 5.92�� | mmol/L | 7.52-11.82 |
3 | �ƣ�Ca�� | 1.68 | mmol/L | 1.55-2.10 |
���� | ||||
�����ϱ������ݣ��ش��������⣺
��1���ö�ͯ_____Ԫ�غ���ƫ�͡�
��2�����浥������mol/L����__________�������������������������Ũ�������ĵ�λ��
��3������ά����C��ʹʳ���е�Fe3+ת��ΪFe2+�����������������ά����C��________��������������������ԭ��������
II. ������أ�K2FeO4����Ԫ��Ϊ+6�ۣ���һ�����͡���Ч�������ɫˮ��������������Ⱦ���Ʊ�������ص����ӷ�Ӧ����ʽΪ��___Fe(OH)3+__ClO-+___OH- = ___FeO42-+___Cl-+ __H2O
�ش��������⣺
��4����ƽ�����ӷ���ʽ��
��5��ÿ����1molFeO42-ת�Ƶĵ�����Ϊ___________������Ӧ������ת����0.3mo1���ӣ���ԭ��������ʵ���Ϊ________mo1��
���𰸡�������Fe�� Ũ�� ��ԭ�� 2 3 4 2 3 5 3NA����1.806��1024 �� 0.15
��������
(1)������챨�浥��֪��(��Fe)Ԫ�غ���ƫ�ͣ�
��2����mol/L��Ũ�ȵĵ�λ��
��3������ά����C��ʹʳ���е�Fe3+ת��ΪFe2+��FeԪ�صĻ��ϼ۽��ͱ���ԭ��
��4����δ��ƽ��ѧ����ʽ��֪��Ӧ��ClԪ�صĻ����ϼ۽��ͣ�FeԪ�صĻ��ϼ����ߣ�Fe(OH)3Ϊ��ԭ����ClO-Ϊ���������ɵ����غ��ԭ���غ�ɵ���ƽ����ʽ��
��5������ƽ�Ļ�ѧ����ʽ����ɵá�
(1)������챨�浥����(��Fe)Ԫ�غ���ƫ�ͣ��ʴ�Ϊ��(��Fe)��
(2)���浥�Ц�mol/L��Ũ�ȵĵ�λ���ʴ�ΪŨ�ȣ�
��3������ά����C��ʹʳ���е�Fe3+ת��ΪFe2+��FeԪ�صĻ��ϼ۽��ͱ���ԭ����ά����C����ԭ���ã��ʴ�Ϊ��ԭ�ԣ�
��4����δ��ƽ��ѧ����ʽ��֪��Ӧ��ClԪ�صĻ����ϼ۽��ͣ�FeԪ�صĻ��ϼ����ߣ�Fe(OH)3Ϊ��ԭ����ClO-Ϊ���������ɵ����غ��ԭ���غ��֪���÷�ӦΪ2Fe��OH��3+3ClO-+4OH-=2FeO42-+3Cl-+5H2O���ʴ�Ϊ2��3��4��2��3��5��
��5������ƽ�Ļ�ѧ����ʽ��֪������2molFeO42-����Ӧת�Ƶĵ�����Ϊ6mol������3mol Cl-����ÿ����1molFeO42-ת�Ƶĵ��ӵ����ʵ���Ϊ3mol����ĿΪ3NA����1.806��1024 ��������Ӧ������ת����0.3mo1���ӣ�������Cl-�����ʵ���Ϊ![]() =0.15mol���ʴ�Ϊ3NA����1.806��1024 ����0.15��
=0.15mol���ʴ�Ϊ3NA����1.806��1024 ����0.15��

����Ŀ���������ƣ���ѧʽΪ NaNO2����һ�ֳ��õķ��������ش���������:
��1��NaNO2 �� N Ԫ�صĻ��ϼ�Ϊ_________.
��2������������ 320��C ʱ�ֽܷ���������ƹ��塢һ��������һ�ֳ�������ȼ�����塣�÷�Ӧ�Ļ�ѧ����ʽ_________________��
��3���ҹ��涨���ȳ��������������ӱ�Ϊÿǧ��ʳƷ���������� 150 ���ˣ��Դ˼��㣬200g 15��������������Һ���ٿ������������ȳ�______ǧ�ˡ�
��4�������������£�NaNO2�밴���ʵ��� 1:1 ǡ����ȫ��Ӧ����I��������Ϊ I2ʱ�������к���������Ϊ________���ѧʽ����
��5����ҵ��ˮ�е� NaNO2 �������۳�ȥ����֪����ϵ�а��� AI��NaAlO2��NaNO2��NaOH��NH3��H2O �������ʡ��÷�Ӧ�Ļ�ѧ����ʽΪ____________��
��6��ijͬѧ���ʵ��Թ�ҵ��Ʒ�� NaNO2 �ĺ������вⶨ����ȡ������Ʒ 2g����ȫ�ܽ����Ƴ���Һ 100mL ȡ�� 25mL ��Һ�� 0.100 mol/L ���� KMnO4 ��Һ���еζ������ʲ��� KMnO4 ��Ӧ����ʵ�������������±���ʾ:
����� | 1 | 2 | 3 | 4 |
����KMnO4��Һ���/mL | 20.70 | 20.02 | 20.00 | 19.98 |
����Ʒ���������Ƶ���������Ϊ_________.����֪:5NO2-+2MnO4-+6H+ = 5NO3-+2Mn2++3H2O��
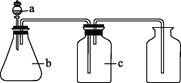
����Ŀ����ͼ��a��b��c��ʾ��Ӧ�����м�����Լ���������ͼװ����ȡ���������ռ���������(����)
��� | ���� | a | b | c |
|
A | NH3 | Ũ��ˮ | ��ʯ�� | ��ʯ�� | |
B | CO2 | ���� | ̼��� | ����NaHCO3��Һ | |
C | NO | ϡ���� | ͭм | H2O | |
D | NO2 | Ũ���� | ͭм | NaOH��Һ |
A. AB. BC. CD. D