��Ŀ����
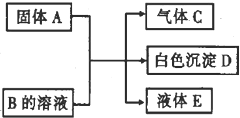 ��2012?��ɫ��ģ��A��B��C��D��Ϊ��ѧ��ѧ�е����ʣ����Ǽ�ķ�Ӧ��ϵ����ͼ��ʾ��
��2012?��ɫ��ģ��A��B��C��D��Ϊ��ѧ��ѧ�е����ʣ����Ǽ�ķ�Ӧ��ϵ����ͼ��ʾ����1����A�ǿ�����ǿ�B�����Σ�D������ϡ���ᣬ��ͼ�з�Ӧ�Ļ�ѧ����ʽΪ
��NH4��2SO4+Ba��OH��2�TBaSO4��+2NH3��+2H2O
��NH4��2SO4+Ba��OH��2�TBaSO4��+2NH3��+2H2O
��C�ķе���Խϸߣ�ԭ�������Ӽ�������
���Ӽ�������
����2����AΪ���ӻ�������廯����CΪֱ���ͷ��ӣ�DΪCaCO3��EΪˮ����B�Ļ�ѧʽ��
Ca��HCO3��2
Ca��HCO3��2
��C�ĽṹʽΪH-C��C-H
H-C��C-H
��52g����C���ȼ�������ȶ�����ʱ���ų�����2600kJ��C��ȼ������1300kJ/mol
1300kJ/mol
����3����A�ǵ���ɫ���壻B�����Σ�B����BaC12��Һ��Ӧ���ɲ�����ϡ����ij�����D�ȿ����������ֿ���
��NaOH��Һ����A�ĵ���ʽ��
��a mol��A�뺬b molB����Һ��ϣ����ɵ�D����ҺE��һ�ֲ������ʺ���ͬ�ֽ���Ԫ�أ��Ҹý���Ԫ�ص����ʵ�����ȣ�����a��b�ı�ֵ��д��������̣���
| 7 |
| 2 |
| 7 |
| 2 |
��������1����A�ǿ�����ǿ�B�����Σ�D������ϡ���ᣬC�����壬ǿ�����η�Ӧ�������就��������C��NH3��D������ϡ���ᣬA��B�������ֽⷴӦ����D��A��ǿ�����A��Ba��OH��2��D��BaSO4��B�����Σ���B�ǣ�NH4��2SO4��E��H2O��
��2����AΪ���ӻ�������廯����CΪֱ���ͷ��ӣ���CΪC2H2��DΪCaCO3��EΪˮ����BΪCa��HCO3��2��A��CaC2��
��3����A�ǵ���ɫ���壬ΪNa2O2��B�����Σ�B����BaCl2��Һ��Ӧ���ɲ�����ϡ����ij�����D�ȿ����������ֿ�����NaOH��Һ��D��Al��OH��3��C��O2������Ԫ���غ�֪��B�к�����Ԫ�أ�BΪ���Σ����ܺ��Ȼ���������ɫ������ϡ����ij̶ȣ���B��Al2��SO4��3��
��2����AΪ���ӻ�������廯����CΪֱ���ͷ��ӣ���CΪC2H2��DΪCaCO3��EΪˮ����BΪCa��HCO3��2��A��CaC2��
��3����A�ǵ���ɫ���壬ΪNa2O2��B�����Σ�B����BaCl2��Һ��Ӧ���ɲ�����ϡ����ij�����D�ȿ����������ֿ�����NaOH��Һ��D��Al��OH��3��C��O2������Ԫ���غ�֪��B�к�����Ԫ�أ�BΪ���Σ����ܺ��Ȼ���������ɫ������ϡ����ij̶ȣ���B��Al2��SO4��3��
����⣺��1����A�ǿ�����ǿ�B�����Σ�D������ϡ���ᣬC�����壬ǿ�����η�Ӧ�������就��������C��NH3��D������ϡ���ᣬA��B�������ֽⷴӦ����D��A��ǿ�����A��Ba��OH��2��D��BaSO4��B�����Σ���B�ǣ�NH4��2SO4��E��H2O��
�÷�Ӧ����ʽΪ����NH4��2SO4+Ba��OH��2�TBaSO4��+2NH3��+2H2O��C�ǰ����������д���������°����е�ϸߣ�
�ʴ�Ϊ����NH4��2SO4+Ba��OH��2�TBaSO4��+2NH3��+2H2O���������Ӽ���������
��2����AΪ���ӻ�������廯����CΪֱ���ͷ��ӣ���CΪC2H2��DΪCaCO3��EΪˮ����BΪCa��HCO3��2��A��CaC2��
ͨ�����Ϸ���֪��B�Ļ�ѧʽ��Ca��HCO3��2��C�ĽṹʽΪH-C��C-H��52g����C�����ʵ���=
=2mol�����ȼ�������ȶ�����ʱ���ų�����2600kJ��C��ȼ������1300kJ/mol��
�ʴ�Ϊ��Ca��HCO3��2��H-C��C-H��1300kJ/mol��
��3����A�ǵ���ɫ���壬ΪNa2O2��B�����Σ�B����BaCl2��Һ��Ӧ���ɲ�����ϡ����ij�����D�ȿ����������ֿ�����NaOH��Һ��D��Al��OH��3��C��O2������Ԫ���غ�֪��B�к�����Ԫ�أ�BΪ���Σ����ܺ��Ȼ���������ɫ������ϡ����ij̶ȣ���B��Al2��SO4��3��
ͨ�����Ϸ���֪��A��Na2O2�������ʽΪ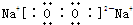 ��
��
��a mol��Na2O2�뺬b molAl2��SO4��3����Һ��ϣ����ɵ�Al��OH��3����ҺE��һ�ֲ������ʺ���ͬ�ֽ���Ԫ�أ��Ҹý���Ԫ�ص����ʵ�����ȣ���E�е�һ�ֲ�����NaAlO2��
Na2O2-----2NaOH
amol 2amol
6NaOH+Al2��SO4��3�T2Al��OH��3��+3Na2SO4
6bmol bmol 2bmol
Al��OH��3 +NaOH�TNaAlO2 +2H2O
��2a-6b��mol ��2a-6b��mol ��2a-6b��mol
2b-��2a-6b��=2a-6b��
=
�ʴ�Ϊ��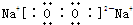 ��
��
��
�÷�Ӧ����ʽΪ����NH4��2SO4+Ba��OH��2�TBaSO4��+2NH3��+2H2O��C�ǰ����������д���������°����е�ϸߣ�
�ʴ�Ϊ����NH4��2SO4+Ba��OH��2�TBaSO4��+2NH3��+2H2O���������Ӽ���������
��2����AΪ���ӻ�������廯����CΪֱ���ͷ��ӣ���CΪC2H2��DΪCaCO3��EΪˮ����BΪCa��HCO3��2��A��CaC2��
ͨ�����Ϸ���֪��B�Ļ�ѧʽ��Ca��HCO3��2��C�ĽṹʽΪH-C��C-H��52g����C�����ʵ���=
| 52g |
| 26g/mol |
�ʴ�Ϊ��Ca��HCO3��2��H-C��C-H��1300kJ/mol��
��3����A�ǵ���ɫ���壬ΪNa2O2��B�����Σ�B����BaCl2��Һ��Ӧ���ɲ�����ϡ����ij�����D�ȿ����������ֿ�����NaOH��Һ��D��Al��OH��3��C��O2������Ԫ���غ�֪��B�к�����Ԫ�أ�BΪ���Σ����ܺ��Ȼ���������ɫ������ϡ����ij̶ȣ���B��Al2��SO4��3��
ͨ�����Ϸ���֪��A��Na2O2�������ʽΪ
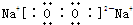 ��
����a mol��Na2O2�뺬b molAl2��SO4��3����Һ��ϣ����ɵ�Al��OH��3����ҺE��һ�ֲ������ʺ���ͬ�ֽ���Ԫ�أ��Ҹý���Ԫ�ص����ʵ�����ȣ���E�е�һ�ֲ�����NaAlO2��
Na2O2-----2NaOH
amol 2amol
6NaOH+Al2��SO4��3�T2Al��OH��3��+3Na2SO4
6bmol bmol 2bmol
Al��OH��3 +NaOH�TNaAlO2 +2H2O
��2a-6b��mol ��2a-6b��mol ��2a-6b��mol
2b-��2a-6b��=2a-6b��
| a |
| b |
| 7 |
| 2 |
�ʴ�Ϊ��
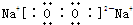 ��
��| 7 |
| 2 |
���������⿼�������ʵ����ʣ���ȷ�ƶ������ǽⱾ��ؼ�����������Ϣ���з�������ѵ��ǣ�3���м���a��b�ı�ֵ�����ԭ���غ㡢��ѧ��Ӧ����ʽ���н���Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
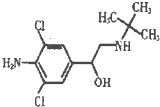 ��2012?��ɫ��ģ�����⾫ѧ������������ޣ���ṹ��ʽ��ͼ�����й������⾫��˵������ȷ����
��2012?��ɫ��ģ�����⾫ѧ������������ޣ���ṹ��ʽ��ͼ�����й������⾫��˵������ȷ����