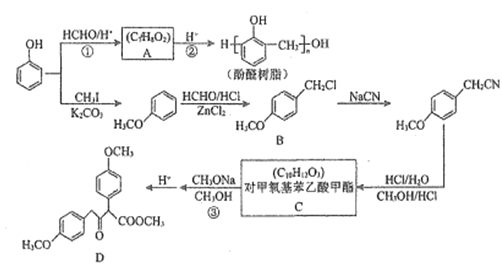
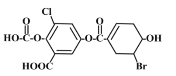
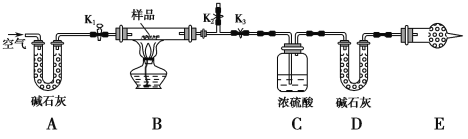
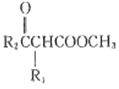��Ŀ����
����Ŀ���Ӷ��Է����������о��Ǹ�������ѧѧϰ����Ҫ��չ���̡����������ѧ֪ʶ�����»�ѧ������ж���������
��1�����������һ�ֵ��͵�ǿ������������KMnO4������Һ����CuS�Ļ����ʱ�������ķ�Ӧ���£�MnO4-+ CuS + H+=Cu2+ + SO2�� + Mn2+�� H2O������ƽ���ӷ���ʽ���õ����ŷ�����÷�Ӧ�е���ת�Ƶķ������Ŀ��___��
��2��K2Cr2O7+14HCl�T2KCl+2CrCl3+3Cl2��+7H2O��������__�����������뻹ԭ��������ʵ���֮��Ϊ___��
��3��Cl2��һ���ж����壬���й©��������صĻ�����Ⱦ������������Ũ��ˮ������Cl2�Ƿ�й©���йط�Ӧ�Ļ�ѧ����ʽΪ��3Cl2������+8NH3������=6NH4Cl���̣�+N2������������Ӧ������Cl2 1.5mol����������NH3�ڱ�״���µ����Ϊ__ L��
���𰸡�![]() K2Cr2O7 3��2 22.4
K2Cr2O7 3��2 22.4
��������
��1������������ԭ��Ӧ�����غ㡢����غ㡢ԭ���غ���ƽ���ӷ���ʽ��
��2�����ݵ����غ���㣻
��3�����ݵ����غ���㡣
��1����Ӧ����MnԪ�ػ��ϼ۽��ͣ���+7�۽���Ϊ+2�ۣ�1mol MnO4-�õ�5mol���ӣ�CuS����Ԫ�ػ��ϼ۴�-2�����ߵ�+4�ۣ�1mol CuSʧȥ6mol���ӣ���������ʵ���֮��Ϊ6��5�����ݵ���غ㣬ԭ���غ���ƽ����ʽ��6MnO4-+5CuS +28 H+=5Cu2++5SO2��+6Mn2++14H2O��ת�Ƶ�����ĿΪ5��6=30���ɱ�ʾΪ ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��2����Ԫ�صĻ��ϼ۽��ͣ�����������K2Cr2O7����������������ʵ�����x mol����ԭ��������ʵ�����y mol�����ݵ����غ��x mol��2=y mol��3�����:x:y=3:2��
��3���豻������NH3�ڱ�״���µ����Ϊx L�����ݵ����غ�ã�1.5mol��2��1=x��22.4L/mol��3����ã�x=22.4���ʴ�Ϊ��22.4��

����Ŀ�����й���ͬ���칹��(�����������칹)����Ŀ�ж���ȷ����( )
ѡ�� | A | B | C | D |
����ʽ(��ṹ��ʽ) | C5H10O2 | C5H10 |
| C7H16 |
��֪���� | ����̼�����Ʒ�Ӧ | ��ʹ�� ˮ��ɫ | һ�ȴ��� | �����к� ��3���� |
ͬ���칹����Ŀ | 4 | 3 | 4 | 5 |
A. AB. BC. CD. D