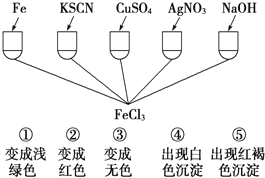
��Ŀ����
����Ŀ����һ��NaCl��Na2CO3��10H2O��NaHCO3�Ļ���ijͬѧ�����ͼ��ʾ��ʵ��װ�ã�ͨ��������Ӧ������CO2��H2O����������ȷ���û�����и���ֵ�����������
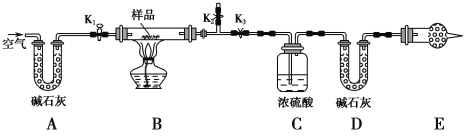
(1) ʵ�鲽�裺
�� ��ͼ(�г�����δ����)��װ��ʵ��װ�ú����Ƚ��еIJ�����____________________��
�� ��ȡ��Ʒ�����������Ӳ�ʲ������У�����װŨ�����ϴ��ƿC��������װ��ʯ�ҵ�U�ι�D��������
�۴���K1��K2���ر�K3������������������ӣ���Ŀ����___________________��
�ܹرջ���K1��K2����K3����ȼ�ƾ��Ƽ��������ٲ������塣װ��B�з�����Ӧ�Ļ�ѧ����ʽΪ___________________________��______________________________��
�� ����K1������������������ӣ�Ȼ�����װ�ã��ٴγ���ϴ��ƿC��������U�ι�D��������
(2) ���ڸ�ʵ�鷽������ش��������⡣
�� E���������ʢ�ŵ�ҩƷ��________����������______________________________________�����ʵ����û�и�װ�ã�����ܵ��²������NaHCO3����������________(����ƫ������ƫС��������Ӱ����)��
�� ����Ʒ����Ϊw g����Ӧ��C��Dװ�����ӵ������ֱ�Ϊm1 g��m2 g����������Na2CO3��10H2O����������Ϊ__________________(�ú�w��m1��m2�Ĵ���ʽ��ʾ)��
���𰸡����װ�������� ��ȥװ���е�ˮ�����Ͷ�����̼ 2NaHCO3![]() Na2CO3��H2O����CO2�� Na2CO3��10H2O
Na2CO3��H2O����CO2�� Na2CO3��10H2O![]() Na2CO3��10H2O�� ��ʯ�� ��ֹ�����е�CO2��ˮ��������D��Ӱ��ⶨ��� ƫ��
Na2CO3��10H2O�� ��ʯ�� ��ֹ�����е�CO2��ˮ��������D��Ӱ��ⶨ��� ƫ�� ![]()
��������
���������Ȼ����H2O��g����CO2���壬Ӧ��C��D�зֱ����գ��ɸ����������֪Ӧ������ˮ�������ն�����̼����C�еĸ������ˮ��������CO2����D�����أ�NaHCO3�ֽ������CO2�������������NaHCO3��������C�����أ�Na2CO310H2O�ֽ������H2O���Ѿ�֪����NaHCO3�ֽ������H2O�������������Na2CO310H2O���������Ӷ����NaCl����������Ӧ��ʵ��ǰ�뷨�ϳ�װ���еĿ������ؼ�����Ӧ�Ǹ�B�еĿ��������Թر�b����a�ͳ�Ϊ�����Ĺؼ�������ͨ������Ϊ�˸ϳ�Ч�����ã�E�м�ʯ�ҿɷ�ֹ�������е�H2O��g����CO2����װ��DӰ��ʵ��Ч�����������Ϸ������н����
��1������ʵ��ԭ����֪��ʵ����Ҫͨ������Dװ���ڼ�ʯ�ҵ����أ��������ɵĶ�����̼��������ͨ������Cװ�õ����أ��������ɵ�ˮ����������Ӧ���ȼ���װ�õ������ԣ���װ�����п���������ˮ�����Ͷ�����̼��Ӱ��ˮ�����Ͷ�����̼�����IJⶨ������K1��K2���رջ���K3��ʵ��ǰҪͨ�����������װ���к���ˮ�����Ͷ�����̼���������ܺ�NaCl��Na2CO310H2O��NaHCO3�Ļ�������ʱ��̼�����Ʒֽ�����̼���ơ�������̼��ˮ��̼���ƾ���ʧȥ�ᾧˮ����̼���ƣ���Ӧ�Ļ�ѧ����ʽΪ��2NaHCO3![]() Na2CO3+H2O��+CO2����Na2CO310H2O
Na2CO3+H2O��+CO2����Na2CO310H2O![]() Na2CO3+10H2O������2������ʵ��ԭ����֪��ʵ����Ҫͨ������Dװ���ڼ�ʯ�ҵ����أ��������ɵĶ�����̼��������ͨ������Cװ��װ�ã��������ɵ�ˮ��������������Ҫ��ֹ�����е�CO2��ˮ��������Dװ�������Ը����E��ʢ�ŵ��Ǽ�ʯ�ң�����ȥEװ�ã���D�����ն�����̼������ƫ�����Բⶨ��̼�����Ƶ�����ƫ����Dװ�������ӵ�����Ϊ������̼��������̼�����Ʒֽ����ɵ�ˮ����������Ϊx��
Na2CO3+10H2O������2������ʵ��ԭ����֪��ʵ����Ҫͨ������Dװ���ڼ�ʯ�ҵ����أ��������ɵĶ�����̼��������ͨ������Cװ��װ�ã��������ɵ�ˮ��������������Ҫ��ֹ�����е�CO2��ˮ��������Dװ�������Ը����E��ʢ�ŵ��Ǽ�ʯ�ң�����ȥEװ�ã���D�����ն�����̼������ƫ�����Բⶨ��̼�����Ƶ�����ƫ����Dװ�������ӵ�����Ϊ������̼��������̼�����Ʒֽ����ɵ�ˮ����������Ϊx��
2NaHCO3![]() Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2��
18g44g
x m2
x=![]() ��װ��C���յ���ˮ����������̼�����Ʒֽ����ɵĺ�Na2CO310H2O�ֽ����ɵģ�Na2CO310H2O�ֽ����ɵ�ˮ����������= m1g-
��װ��C���յ���ˮ����������̼�����Ʒֽ����ɵĺ�Na2CO310H2O�ֽ����ɵģ�Na2CO310H2O�ֽ����ɵ�ˮ����������= m1g-![]() g����Na2CO310H2O������Ϊy��
g����Na2CO310H2O������Ϊy��
Na2CO310H2O![]() Na2CO3+10H2O
Na2CO3+10H2O
286g 180g
y m1g-![]() g
g
y=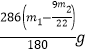 ������Na2CO310H2O����������=
������Na2CO310H2O����������=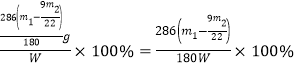 ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����һ�����ܱ�������,������Ӧ:CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H����ƽ�ⳣ��(K)���¶�(T)�Ĺ�ϵ���±�:
CO(g)+H2O(g) ��H����ƽ�ⳣ��(K)���¶�(T)�Ĺ�ϵ���±�:
T�� | 700 | 800 | 850 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�Իش���������:
��1��������ӦΪ____(��������������������)��Ӧ,�����¶�,ƽ����______ (��������Ӧ�������淴Ӧ��) �����ƶ���
��2��ij�¶���,�����Ϊ2L�ĺ����ܱ�������ͨ��2molCO2(g)��4molH2(B)��������Ӧ,5minʱ��Ӧ�ﵽƽ��,���CO2(g)��ת������75%��
��v(H2O)=______mol��L-1��min-l��
�ڸ��¶��·�Ӧ��ƽ�ⳣ��K=______.
��3������ˮú���Ĺ�������:
��C(s)+CO2(g) ![]() 2CO(g)��H1
2CO(g)��H1
��CO(g)��H2O(g)![]() CO2(g)��H2(g) ��H2
CO2(g)��H2(g) ��H2
�۷�Ӧ:CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H=________ (�ú���H1����H2�Ĵ���ʽ��ʾ)��
CO(g)+H2O(g) ��H=________ (�ú���H1����H2�Ĵ���ʽ��ʾ)��
����Ŀ��ӡȾ��ҵ������������(NaClO2)Ư��֯��ù������ⷨ�����������ƵĹ�����������:
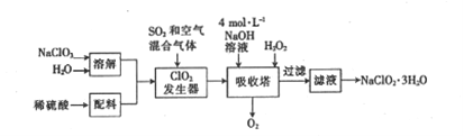
��֪:��NaClO2���ܽ�����¶ȵ����߶�����,�ʵ������¿ɽᾧ����NaClO2��3H2O��
��Ksp(FeS)=6.3��10-18;Ksp(CuS)= 6.3��10-36;Ksp(PbS)=2.4��10-28��
��1���������з�Ӧ�Ļ�ԭ����_________(�ѧʽ����ͬ)����������ClO2�ڼ�����������H2O2����NaClO2 �����ӷ���ʽΪ_______
��2������Һ�еõ���NaClO2��3H2O�IJ���������_______��(����ĸ)��ϴȾ�����
a.���� b.���� c.��ȴ�ᾧ d.����Ũ�� e.����
��3����������Ư��֯��ʱ���������õ���HClO2���±���25��ʱHClO2�����ֳ�������ĵ���ƽ�ⳣ����
���� | HClO2 | HF | HCN | H2S |
Ka/mol��L-1 | 1��10-2 | 6.3��10-4 | 4.9��10-10 | K1=9.1��10-8 K2=1.1��10-12 |
�ٳ����£����ʵ���Ũ����ͬ��NaF��NaCN ������Һ��PH�ɴ�С��˳��Ϊ________��
�ڵ���������ʵ���Ũ�ȵ�HClO2��NaOH��Һ��ַ�Ӧ��,��Һ�и�����Ũ���ɴ�С��˳��Ϊ_____��
��Na2S�dz��õij�������ij��ҵ��ˮ�к��е�Ũ�ȵ�Cu2+��Fe2+��Pb2+,�μ�Na2S��Һ�����������ij�����______;�����һ�����ӳ�����ȫʱ(������Ũ��Ϊ10-5 mol��L-l),��ʱ��ϵ��S2-��Ũ��Ϊ________��