��Ŀ����
����Ŀ�����ܱ������з������·�Ӧ��aA(g)+bB(g)![]() cC(g)��������������ʱ���ı�ijһ������������Ӧ��Ӱ�죬�õ�����ͼ��(ͼ��p��ʾѹǿ��T��ʾ�¶ȣ�n��ʾ���ʵ���������ʾƽ��ת����)�����з�����ȷ����
cC(g)��������������ʱ���ı�ijһ������������Ӧ��Ӱ�죬�õ�����ͼ��(ͼ��p��ʾѹǿ��T��ʾ�¶ȣ�n��ʾ���ʵ���������ʾƽ��ת����)�����з�����ȷ����
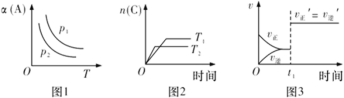
A.��ͼ1��Ӧ����p1>p2����÷�Ӧֻ���ڸ������Է�����
B.��ͼ2��Ӧ���˷�Ӧ�ġ�H<0����T1>T2
C.��ͼ3��Ӧ����ʾt1ʱ�̿�����ʹ�ô����Է�Ӧ���ʵ�Ӱ��
D.��ͼ3��Ӧ����ʾt1ʱ������B��Ũ�ȶԷ�Ӧ���ʵ�Ӱ��
���𰸡�C
��������
A���ɷ�Ӧ1ͼ��֪������ѹǿ��A��ת��������ƽ�������ƶ���˵������Ӧ���ؼ��ķ�Ӧ������ƽ�������ƶ���˵������Ӧ�Ƿ��ȷ�Ӧ��������G=��H-T��S��֪���÷�Ӧ�ڵ��¶�ʱ�������Է����У���A����
B����ͼ��֪��T2����ʼб�ʴ���T1��˵��T1��T2�������¶������ﺬ����С��˵��ƽ�������ƶ���������ӦΪ���ȷ�Ӧ����B����
C������Ӧ�Ƿ�Ӧǰ�������ϵ���Ͳ���ķ�Ӧ������ѹǿ�ͼӴ�������������ѧƽ����ƶ���t1ʱ�̿���������ѹǿ���ǼӴ������������C��ȷ��
D������B��Ũ��˲�䣬����Ӧ��������ƽ�������ƶ���������ͼ��D����
�ʴ�ΪC��

 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
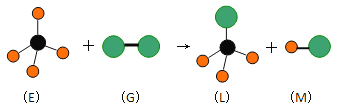
������ҵ��ٳɳ����½������������ϵ�д�����Ŀ����֪������ijЩ�л����ģ��ͼ������Ҫ��ش��������⣺
��� | �� | �� | �� |
ģ�� |
|
|
|
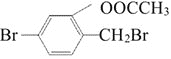
(1)�ķ���ʽ��___________________����һ�ȴ�����___________________�֡�
(2)�����![]() ��
��![]() ��Һ������Ӧ�Ļ�ѧ����ʽ��______________________________________���÷�Ӧ�ķ�Ӧ������___________________��
��Һ������Ӧ�Ļ�ѧ����ʽ��______________________________________���÷�Ӧ�ķ�Ӧ������___________________��
(3)����Ħ������Ϊ![]() �����ñ��Ʊ�������ʱ�Ĵ�����___________________��
�����ñ��Ʊ�������ʱ�Ĵ�����___________________��