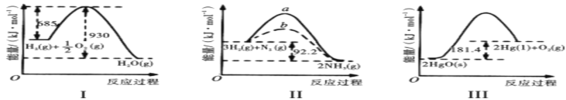
��Ŀ����
����Ŀ��������(AlN)�մ���һ���������ǽ������ϣ� ��߿��ȶ���2473K�� �����Ժá�������ϵ��С�������õ����ȳ�����ϡ���ȡԭ��Ϊ��Al2O3+3C+N2![]() 2A1N+3CO���ش��������⣺
2A1N+3CO���ش��������⣺
(1)�������ľ�������Ϊ________����������ѧ����ʽ�еڶ�����Ԫ�صĵ�һ��������С�����˳����______��
(2)��̬��ԭ�ӵ���ռ������ܼ���ԭ�ӹ������״��________��δ�ɶԵ�����Ϊ________��
(3)�ȵ�����������ƵĽṹ��CO��N2��Ϊ�ȵ����壬CO������������������Ŀ֮��Ϊ_______��
(4)Cu2+���ڣ�[Cu(NH3)4]2+�����ģ�����������[Cu(NH3)4]2+�е�2��NH3��ΪCN-������2�ֽṹ����Cu2+�Ƿ�Ϊsp3�ӻ�________������������������������Ϊ_________��
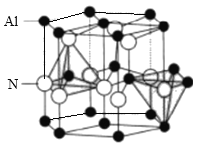
(5)AlN����ṹ��ͼ��ʾ��1��Alԭ����Χ���������Alԭ����Ϊ______�����������ṹ�ĸ�Ϊa nm�� �ױ߳�Ϊb nm��NA��ʾ����٤��������ֵ�������ܶ�Ϊ_______g.cm-3(�г�����ʽ)��
���𰸡�ԭ�Ӿ��� C<O<N ������(��Ĵ���) 2 1��2 �� ����sp3�ӻ�[Cu(NH3)4]2+�Ŀռ乹��Ϊ���������Σ���������[Cu(NH3)4]2+�е�2��NH3��ΪCN-����ֻ��1�ֽṹ 12 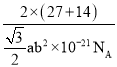
��������
(1)ԭ�Ӿ�����۷е�ϸߡ�Ӳ�ȴ�ͬһ����Ԫ�صĵ����ܳ��������ƣ�
(2)����Oԭ�Ӻ�������Ų��������ܼ���ԭ�ӹ������״ȷ��δ�ɶԵ�����Ŀ��
(3)�ȵ�����ṹ���ƣ���Ϲ��۵�����������������������˫��һ����������1������������������1��������2������������
(4)���ݽ�������[Cu(NH3)4]2+�е�2��NH3��ΪCN-����2�ֽṹ���ж�Cu2+��ԭ���ӻ����ͣ�
(5)���þ�̯��������һ�������к��е���Alԭ�������Alԭ�Ӹ������ȼ���һ�������к��е�Al��Nԭ����Ŀ��Ȼ����ݾ����ܶȼ��㹫ʽ![]() ���㡣
���㡣
(1)����ԭ�Ӿ�����۷е�ϸߡ�Ӳ�ȴ�������(AlN)�մ���߿��ȶ���2473K��˵��ԭ�Ӽ�����ǿ���۷е�ߣ�����ԭ�Ӿ��壻��������Ӧ���漰���ĵڶ����ڵ�Ԫ����C��N��O����Ԫ�أ�ͬһԪ�صĵ�������ԭ���������������������Nԭ��������p���Ӵ��ڰ�������ȶ�״̬��������ʧȥ���ӣ��������һ�����ܱ����ڵ�OԪ��Ҫ������Ԫ�صĵ�һ��������С�����˳����C<O<N��
(2)O��8��Ԫ�أ���������Ų�Ϊ1s22s22p4���ɼ���̬��ԭ�ӵ���ռ������ܼ���2p�ܼ�����ԭ�ӹ������״��������(��Ĵ���)������2p�����Ŀ��3 ����ԭ�Ӻ���������Ǿ����ܳɵ����У���������������ͬ��һ�������������2�����ӣ����������෴������δ�ɶԵ�����Ϊ2����
(3) N2�ṹ��ʽ��N��N���ȵ�����������ƵĽṹ��CO��N2��Ϊ�ȵ����壬����CO�����к���1��������2�����������CO������������������Ŀ֮��Ϊ1��2��
(4)���ڽ�������[Cu(NH3)4]2+�е�2��NH3��ΪCN-����2�ֽṹ��˵��[Cu(NH3)4]2+�γ���ƽ�������νṹ��Cu2+��ƽ�������ζԽ��ߵĽ����ϣ�����Cu2+����sp3�ӻ����������������κ��������㶼��������λ�ã���ô[Cu(NH3)4]2+�Ŀռ乹��Ϊ���������Σ���������[Cu(NH3)4]2+�е�2��NH3��ΪCN-����ֻ��1�ֽṹ������Cu2+���Dz���sp3�ӻ���
(5)�ɾ���ṹʾ��ͼ��֪��Alԭ�����ӵ�Nԭ�ӹ��ɵ�����������ṹ����Nԭ�����ӵ�Alԭ�ӹ��ɵ�Ҳ����������ṹ����������Alԭ�Ӵ��������嶥���ϣ��ɽ�ͼΪ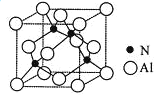 ���ɼ�����Alԭ�ӵ��������Alԭ���������һ����������3������Alԭ�ӣ���ͨ����Alԭ�ӿ��γ�8��������ÿ��Alԭ�ӱ�������2�Σ������1��Alԭ����Χ���������Alԭ����Ϊ
���ɼ�����Alԭ�ӵ��������Alԭ���������һ����������3������Alԭ�ӣ���ͨ����Alԭ�ӿ��γ�8��������ÿ��Alԭ�ӱ�������2�Σ������1��Alԭ����Χ���������Alԭ����Ϊ![]() =12����Ҳ��������ͼ��ʵ�߲��ֽ�ͼΪ
=12����Ҳ��������ͼ��ʵ�߲��ֽ�ͼΪ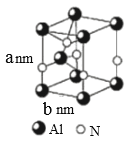 ���ڸýṹͼ�У�����Ϊ���Σ����ڶ�λ���������Ƿֱ�Ϊ60�㡢120�㣬���к���Alԭ�Ӹ���ΪAl��
���ڸýṹͼ�У�����Ϊ���Σ����ڶ�λ���������Ƿֱ�Ϊ60�㡢120�㣬���к���Alԭ�Ӹ���ΪAl��![]() =2������Nԭ����ĿΪN��
=2������Nԭ����ĿΪN��![]() =2�����һ���ýṹ�к���2��AlN���������ΪS=
=2�����һ���ýṹ�к���2��AlN���������ΪS=![]() b��(10-7 cm) b��(10-7 cm)=
b��(10-7 cm) b��(10-7 cm)=![]() b2��10span>-14 cm2��������Ϊa nm=a��10-7 cm����ýṹ���V=
b2��10span>-14 cm2��������Ϊa nm=a��10-7 cm����ýṹ���V=![]() b2��10-21 cm3���ýṹ����m=
b2��10-21 cm3���ýṹ����m=![]() g�����Ըýṹ(������)���ܶ�Ϊ
g�����Ըýṹ(������)���ܶ�Ϊ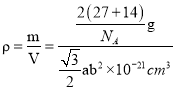 =
=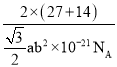 g/cm3��
g/cm3��