ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΒΣ(N)ΓΔΝΉ(P)ΓΔ…ι(As)Β»VAΉε‘ΣΥΊΜ·ΚœΈο‘Ύ―–ΨΩΚΆ…ζ≤ζ÷–”–÷Ί“Σ”ΟΆΨΓΘ»γΈ“ΙζΩΤ―–»Υ‘±―–ΨΩΖΔœ÷As2O3(Μρ–¥≥…As4O6Θ§ΥΉ≥Τ≈χΥΣ)Ε‘ΑΉ―Σ≤Γ”–Οςœ‘ΒΡ÷ΈΝΤΉς”ΟΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©As‘≠Ή”ΒΡΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ_________________ΘΜPΒΡΒΎ“ΜΒγάκΡή±»S¥σΒΡ‘≠“ρΈΣ_______________
Θ®2Θ©–¥≥ω“Μ÷÷”κCNΓΞΜΞΈΣΒ»ΒγΉ”ΧεΒΡΝΘΉ”________(”ΟΜ·―ß Ϋ±μ Ψ)ΘΜ(SCN)2Ζ÷Ή”÷–Π“ΦϋΚΆΠ–ΦϋΗω ΐ±»ΈΣ___________
Θ®3Θ©≈χΥΣΨγΕΨΘ§Ω…”Ο ·Μ“œϊΕΨ…ζ≥…AsO33-ΚΆ…ΌΝΩAsO43-Θ§Τδ÷–AsO33-÷–AsΒΡ‘”Μ·ΖΫ ΫΈΣ__________Θ§AsO43-ΒΡΩ’ΦδΙΙ–ΆΈΣ___________
Θ®4Θ©NH4+÷–H“ΜN“ΜHΒΡΦϋΫ«±»NH3÷–H “ΜN“ΜHΒΡΦϋΫ«¥σΒΡ‘≠“ρ «__________ΘΜNH3ΚΆΥ°Ζ÷Ή””κΆ≠άκΉ”–Έ≥…ΒΡΜ·ΚœΈο÷–―τάκΉ”≥ ÷αœρœΝ≥ΛΒΡΑΥΟφΧεΫαΙΙ(»γœ¬ΆΦI)Θ§ΗΟΜ·ΚœΈοΦ”»» ± Ήœ» ß»ΞΥ°Θ§«κ¥”‘≠Ή”ΫαΙΙΫ«Ε»Φ”“‘Ζ÷ΈωΘΚ__________
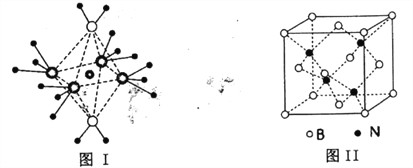
Θ®5Θ©BNΒΡ»έΒψΈΣ3000ΓφΘ§ΟήΕ»ΈΣ2.25gΓΛcm-3Θ§ΤδΨßΑϊΫαΙΙ»γ…œΆΦΔρΥυ ΨΘ§ΨßΧε÷–“ΜΗωB‘≠Ή”÷ήΈßΨύάκΉνΫϋΒΡN‘≠Ή””–__________ΗωΘΜ»τ‘≠Ή”ΑκΨΕΖ÷±πΈΣrNpmΚΆrBpmΘ§ΑΔΖϋΦ”Β¬¬ό≥Θ ΐ÷ΒΈΣNAΘ§‘ρBNΨßΑϊ÷–‘≠Ή”ΒΡΧεΜΐ’ΦΨßΑϊΧεΜΐΒΡΑΌΖ÷¬ ΈΣ__________
ΓΨ¥πΑΗΓΩ 1s22s22p63s23p63d104s24p3Μρ[Ar]3d104s24p3 PΒΡpΡήΦΕ «Ακ≥δ¬ζΉ¥Χ§Θ§±»ΫœΈ»Ε®Θ§Υυ“‘ΒΎ“ΜΒγάκΡή±»Νρ¥σ COΜρN2 5:4 sp3 ’ΐΥΡΟφΧε NH4+÷–ΒΡΒΣ‘≠Ή”…œΨυΈΣ≥…ΦϋΒγΉ”Θ§ΕχNH3Ζ÷Ή”÷–ΒΡΒΣ‘≠Ή”…œ”–“ΜΕ‘Ι¬Ε‘ΒγΉ”Θ§Ι¬Ε‘ΒγΉ””κ≥…ΦϋΒγΉ”÷°ΦδΒΡ≈≈≥βΝΠ«Ω”Ύ≥…ΦϋΒγΉ””κ≥…ΦϋΒγΉ”÷°ΦδΒΡ≈≈≥βΝΠΘ§ΒΦ÷¬NH4+÷–H-N-HΒΡΦϋΫ«±»NH3÷–¥σ ”…”ΎO‘≠Ή”ΑκΨΕ–ΓΘ§ΒγΗΚ–‘¥σΘ§ΧαΙ©Ι¬Ε‘ΒγΉ”ΡήΝΠ±»N‘≠Ή”»θΘ§Ι Υ°Ζ÷Ή”–Έ≥…ΒΡ≈δΈΜΦϋ»θ”ΎΑ±Ζ÷Ή” 4 ![]()
ΓΨΫβΈωΓΩ ‘ΧβΖ÷ΈωΘΚ±ΨΧβΩΦ≤ι‘≠Ή”ΚΥΆβΒγΉ”≈≈≤Φ ΫΒΡ ι–¥Θ§ΒΎ“ΜΒγάκΡήΒΡ±»ΫœΘ§Β»ΒγΉ”ΧεΘ§Π“ΦϋΚΆΠ–ΦϋΒΡ≈–ΕœΘ§‘”Μ·ΖΫ ΫΚΆΩ’ΦδΙΙ–ΆΒΡ≈–ΕœΘ§ΨßΧεΒΡΖ÷ΈωΚΆΦΤΥψΓΘ
Θ®1Θ©As‘≠Ή”ΚΥΆβ”–33ΗωΒγΉ”Θ§ΗυΨίΙΙ‘λ‘≠άμΘ§As‘≠Ή”ΒΡΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ1s22s22p63s23p63d104s24p3Μρ[Ar] 3d104s24p3ΓΘPΚΆSΕΦ¥Π”ΎΒΎ»ΐ÷ήΤΎΘ§SΒΡΦέΒγΉ”≈≈≤Φ ΫΈΣ3s23p4Θ§PΒΡΦέΒγΉ”≈≈≤Φ ΫΈΣ3s23p3Θ§PΒΡ3pΡήΦΕ¥Π”ΎΑκ≥δ¬ζΘ§±»ΫœΈ»Ε®Θ§PΒΡΒΎ“ΜΒγάκΡή±»SΒΡ¥σΓΘ
Θ®2Θ©”ΟΓΑΧφ¥ζΖ®Γ±Θ§”κCN-ΜΞΈΣΒ»ΒγΉ”ΧεΒΡΝΘΉ””–COΓΔN2ΓΔC22-Β»ΓΘΘ®SCNΘ©2ΒΡΫαΙΙ ΫΈΣN![]() CΓΣSΓΣSΓΣC
CΓΣSΓΣSΓΣC![]() NΘ§ΒΞΦϋ»Ϊ «Π“ΦϋΘ§»ΐΦϋ÷–”–1ΗωΠ“ΦϋΚΆ2ΗωΠ–ΦϋΘ§Θ®SCNΘ©2Ζ÷Ή”÷–”–5ΗωΠ“ΦϋΓΔ4ΗωΠ–ΦϋΘ§Π“ΦϋΚΆΠ–ΦϋΗω ΐ±»ΈΣ5:4ΓΘ
NΘ§ΒΞΦϋ»Ϊ «Π“ΦϋΘ§»ΐΦϋ÷–”–1ΗωΠ“ΦϋΚΆ2ΗωΠ–ΦϋΘ§Θ®SCNΘ©2Ζ÷Ή”÷–”–5ΗωΠ“ΦϋΓΔ4ΗωΠ–ΦϋΘ§Π“ΦϋΚΆΠ–ΦϋΗω ΐ±»ΈΣ5:4ΓΘ
Θ®3Θ©AsO33-÷–As…œΒΡΙ¬ΒγΉ”Ε‘ ΐ=![]() Θ®5+3-3
Θ®5+3-3![]() 2Θ©=1Θ§≥…ΦϋΒγΉ”Ε‘ ΐΈΣ3Θ§AsΒΡΦέ≤ψΒγΉ”Ε‘ ΐΈΣ1+3=4Θ§AsΒΡ‘”Μ·ΖΫ ΫΈΣsp3‘”Μ·ΓΘAsO43-÷–As…œΒΡΙ¬ΒγΉ”Ε‘ ΐ=
2Θ©=1Θ§≥…ΦϋΒγΉ”Ε‘ ΐΈΣ3Θ§AsΒΡΦέ≤ψΒγΉ”Ε‘ ΐΈΣ1+3=4Θ§AsΒΡ‘”Μ·ΖΫ ΫΈΣsp3‘”Μ·ΓΘAsO43-÷–As…œΒΡΙ¬ΒγΉ”Ε‘ ΐ=![]() Θ®5+3-4
Θ®5+3-4![]() 2Θ©=0Θ§≥…ΦϋΒγΉ”Ε‘ ΐΈΣ4Θ§AsΒΡΦέ≤ψΒγΉ”Ε‘ ΐΈΣ0+4=4Θ§AsO43-ΒΡVSEPRΡΘ–ΆΈΣ’ΐΥΡΟφΧεΘ§”…”ΎΟΜ”–Ι¬ΒγΉ”Ε‘Θ§AsO43-ΒΡΩ’ΦδΙΙ–ΆΈΣ’ΐΥΡΟφΧεΓΘ
2Θ©=0Θ§≥…ΦϋΒγΉ”Ε‘ ΐΈΣ4Θ§AsΒΡΦέ≤ψΒγΉ”Ε‘ ΐΈΣ0+4=4Θ§AsO43-ΒΡVSEPRΡΘ–ΆΈΣ’ΐΥΡΟφΧεΘ§”…”ΎΟΜ”–Ι¬ΒγΉ”Ε‘Θ§AsO43-ΒΡΩ’ΦδΙΙ–ΆΈΣ’ΐΥΡΟφΧεΓΘ
Θ®4Θ©NH4+÷–HΓΣNΓΣHΒΡΦϋΫ«±»NH3÷–HΓΣNΓΣHΒΡΦϋΫ«¥σΒΡ‘≠“ρ «ΘΚNH4+÷–ΒΡΒΣ‘≠Ή”…œΨυΈΣ≥…ΦϋΒγΉ”Θ§ΕχNH3Ζ÷Ή”÷–ΒΡΒΣ‘≠Ή”…œ”–“ΜΕ‘Ι¬Ε‘ΒγΉ”Θ§Ι¬Ε‘ΒγΉ””κ≥…ΦϋΒγΉ”÷°ΦδΒΡ≈≈≥βΝΠ«Ω”Ύ≥…ΦϋΒγΉ””κ≥…ΦϋΒγΉ”÷°ΦδΒΡ≈≈≥βΝΠΘ§ΒΦ÷¬NH4+÷–H-N-HΒΡΦϋΫ«±»NH3÷–¥σΓΘNH3ΚΆΥ°Ζ÷Ή””κΆ≠άκΉ”–Έ≥…ΒΡΜ·ΚœΈο÷–―τάκΉ”≥ ÷αœρœΝ≥ΛΒΡΑΥΟφΧεΫαΙΙΘ§ΗΟΜ·ΚœΈοΦ”»» ± Ήœ» ß»ΞΥ°Θ§ΥΒΟςΥ°Ζ÷Ή””κCu2+–Έ≥…ΒΡ≈δΈΜΦϋ»θ”ΎNH3Ζ÷Ή”Θ§‘≠“ρ «ΘΚO‘≠Ή”ΑκΨΕ–ΓΘ§ΒγΗΚ–‘¥σΘ§ΧαΙ©Ι¬Ε‘ΒγΉ”ΡήΝΠ±»N‘≠Ή”»θΘ§Ι Υ°Ζ÷Ή””κCu2+–Έ≥…ΒΡ≈δΈΜΦϋ»θ”ΎΑ±Ζ÷Ή”ΓΘ
Θ®5Θ©”ΟΓΑΨυΧ·Ζ®Γ±Θ§BΘΚ8![]() +6
+6![]() =4Θ§NΘΚ4Θ§ΗΟΨßΧεΒΡΜ·―ß ΫΈΣBNΘ§ΗυΨίΨßΑϊΘ§ΨßΧε÷–“ΜΗωN‘≠Ή”÷ήΈßΨύάκΉνΫϋΒΡB‘≠Ή””–4ΗωΘ§‘ρ“ΜΗωB‘≠Ή”÷ήΈßΨύάκΉνΫϋΒΡN‘≠Ή””–4ΗωΓΘ“ΜΗωΨßΑϊ÷–‘≠Ή”ΒΡΧεΜΐΈΣ4
=4Θ§NΘΚ4Θ§ΗΟΨßΧεΒΡΜ·―ß ΫΈΣBNΘ§ΗυΨίΨßΑϊΘ§ΨßΧε÷–“ΜΗωN‘≠Ή”÷ήΈßΨύάκΉνΫϋΒΡB‘≠Ή””–4ΗωΘ§‘ρ“ΜΗωB‘≠Ή”÷ήΈßΨύάκΉνΫϋΒΡN‘≠Ή””–4ΗωΓΘ“ΜΗωΨßΑϊ÷–‘≠Ή”ΒΡΧεΜΐΈΣ4![]() Θ®
Θ®![]() Π–rN3+
Π–rN3+![]() Π–rB3Θ©pm3=4
Π–rB3Θ©pm3=4Θ®
![]() Π–rN3+
Π–rN3+![]() Π–rB3Θ©
Π–rB3Θ©![]() 10-30cm3Θ§1molΨßΧεΒΡ÷ ΝΩΈΣ25gΘ§1molΨßΧεΒΡΧεΜΐΈΣ25g
10-30cm3Θ§1molΨßΧεΒΡ÷ ΝΩΈΣ25gΘ§1molΨßΧεΒΡΧεΜΐΈΣ25g![]() 2.25g/cm3Θ§ΨßΑϊΒΡΧεΜΐΈΣ4
2.25g/cm3Θ§ΨßΑϊΒΡΧεΜΐΈΣ4![]() Θ®25g
Θ®25g![]() 2.25g/cm3
2.25g/cm3![]() NAΘ©Θ§BNΨßΑϊ÷–‘≠Ή”ΒΡΧεΜΐ’ΦΨßΑϊΧεΜΐΒΡΑΌΖ÷¬ ΈΣ4
NAΘ©Θ§BNΨßΑϊ÷–‘≠Ή”ΒΡΧεΜΐ’ΦΨßΑϊΧεΜΐΒΡΑΌΖ÷¬ ΈΣ4![]() Θ®
Θ®![]() Π–rN3+
Π–rN3+![]() Π–rB3Θ©
Π–rB3Θ©![]() 10-30cm3
10-30cm3![]() [4
[4![]() Θ®25g
Θ®25g![]() 2.25g/cm3
2.25g/cm3![]() NAΘ©]=
NAΘ©]=![]() ΓΘ
ΓΘ

 ÷–ΩΦΫβΕΝΩΦΒψΨΪΝΖœΒΝ–¥πΑΗ
÷–ΩΦΫβΕΝΩΦΒψΨΪΝΖœΒΝ–¥πΑΗΓΨΧβΡΩΓΩI.≥ΘΈ¬œ¬Θ§ΫΪΡ≥“Μ‘ΣΥαHAΚΆNaOH»ή“ΚΒ»ΧεΜΐΜλΚœΘ§Ζ÷±πΫχ––±ύΚ≈ΈΣΔΌΓΔΔΎΓΔΔέΒΡ Β―ιΘ§ Β―ι ΐΨίΦ«¬Φ»γœ¬±μΓΘ
–ρΚ≈ | HAΈο÷ ΒΡΝΩ≈®Ε»Θ®molL-1Θ© | NaOHΈο÷ ΒΡΝΩ≈®Ε»Θ®molL-1Θ© | ΜλΚœ»ή“ΚΒΡpH |
ΔΌ | 0.1 | 0.1 | pH=9 |
ΔΎ | c | 0.2 | pH=7 |
Δέ | 0.2 | 0.1 | pH<7 |
Θ®1Θ©ΗυΨίΔΌΉι Β―ι«ιΩωΘ§Ζ÷ΈωΜλΚœ»ή“ΚΒΡpH=9ΒΡ‘≠“ρ «______________________Θ®”ΟάκΉ”ΖΫ≥Χ Ϋ±μ ΨΘ©Θ§‘ΎΗΟ»ή“Κ÷–œ¬Ν–ΙΊœΒ Ϋ¥μΈσΒΡ «________________Θ®Χν±ξΚ≈Θ©ΓΘ
a.c(Na+)+c(H+)=c(OH-)+c(A-) b.c(Na+)=c(HA)+c(A-)
c.c(HA)+c(H+)=c(OH-) d.c(Na+)+c(H+)=c(OH-)+c(A-)+c(HA)
Θ®2Θ©ΔΎΉι«ιΩω±μΟςΘ§c______0.2Θ®ΧνΓΑ>Γ±ΓΑ<Γ±ΜρΓΑ=Γ±Θ§œ¬Ά§Θ©Θ§ΜλΚœ»ή“Κ÷–ΘΚc(A-)_______c(Na+)ΓΘ
Θ®3Θ©¥”ΔέΉι Β―ιΫαΙϊΖ÷ΈωΘ§ΥΒΟςHAΒΡΒγάκ≥ΧΕ»_____NaAΒΡΥ°Ϋβ≥ΧΕ»Θ®ΧνΓΑ>Γ±ΓΑ<Γ±ΜρΓΑ=Γ±Θ©Θ§άκΉ”≈®Ε»”…¥σΒΫ–ΓΒΡΥ≥–ρ «_______________________________ΓΘ
Δρ. “Έ¬œ¬Θ§»τΫΪ0.1 molL-1―ΈΥαΒΈ»κ20mL0.1 molL-1Α±Υ°÷–Θ§»ή“ΚpHΥφΦ”»κ―ΈΥαΧεΜΐΒΡ±δΜ·«ζœΏ»γœ¬ΆΦΥυ ΨΘΚ

Θ®1Θ©NH3ΓΛH2OΒΡΒγάκΖΫ≥Χ Ϋ «_______________________________ΓΘ
Θ®2Θ©bΒψΥυ Ψ»ή“Κ÷–ΒΡ»ή÷ «_______________________________ΓΘ
Θ®3Θ©cΒψΥυ Ψ»ή“Κ÷–Θ§άκΉ”≈®Ε»¥”¥σΒΫ–ΓΒΡΙΊœΒΈΣ_______________________________ΓΘ
ΓΨΧβΡΩΓΩ(1)ΧΦΥαΘΚH2CO3Θ§Ki1=4.3ΓΝ10-7Θ§Ki2=5.6ΓΝ10-11
≤ίΥαΘΚH2C2O4Θ§Ki1=5.9ΓΝ10-2Θ§Ki2=6.4ΓΝ10-5
ΔΌ0.1 mol/L Na2CO3»ή“ΚΒΡpH____________0.1 mol/L Na2C2O4»ή“ΚΒΡpHΓΘΘ®―ΓΧνΓΑ¥σ”ΎΓ±ΓΑ–Γ”ΎΓ±ΜρΓΑΒ»”ΎΓ±Θ©
ΔΎ»τΫΪΒ»≈®Ε»ΒΡ≤ίΥα»ή“ΚΚΆΧΦΥα»ή“ΚΒ»ΧεΜΐΜλΚœΘ§»ή“Κ÷–Ης÷÷άκΉ”≈®Ε»¥σ–ΓΒΡΥ≥–ρ’ΐ»ΖΒΡ «_____ΓΘΘ®―ΓΧν±ύΚ≈Θ©
AΘ°[H+]>[HC2O4-]>[HCO3-]>[CO32-] BΘ°[HCO3-]>[HC2O4-]>[C2O42-]>[CO32-]
CΘ°[H+]>[HC2O4-]>[C2O42-]>[CO32-] DΘ°[H2CO3] >[HCO3-]>[HC2O4-]>[CO32-]
(2)ΑΉΝΉ”κ―θΤχΩ…ΖΔ…ζ»γœ¬Ζ¥”ΠΘΚP4+5O2= P4O10ΓΘ“―÷ΣΕœΝ―œ¬Ν–Μ·―ßΦϋ–η“ΣΈϋ ’ΒΡΡήΝΩΖ÷±πΈΣΘΚ PΓΣP a kJΓΛmol-1ΓΔPΓΣO b kJΓΛmol-1ΓΔPΘΫO c kJΓΛmol-1ΓΔOΘΫO d kJΓΛmol-1ΓΘΗυΨίΆΦ ΨΒΡΖ÷Ή”ΫαΙΙΚΆ”–ΙΊ ΐΨίΙάΥψΗΟΖ¥”ΠΒΡΠΛH=___________ΓΘ

(3)25 ΓφΘ§Ρ≥≈®Ε»ΒΡ―ΈΥαΓΔ¬»Μ·οß»ή“Κ÷–”…Υ°Βγάκ≥ωΒΡ«βάκΉ”≈®Ε»Ζ÷±πΈΣ1.0ΓΝ10Θ≠amolΓΛLΘ≠1ΓΔ1.0ΓΝ10Θ≠b molΓΛLΘ≠1Θ§’βΝΫ÷÷»ή“ΚΒΡpH÷°ΚΆ=___________ΓΘ
(4)‘Ύt Γφ ±Θ§Ρ≥Ba(OH)2ΒΡœΓ»ή“Κ÷–c(HΘΪ)ΘΫ10Θ≠a mol/LΘ§c(OHΘ≠)ΘΫ10Θ≠b mol/LΘ§“―÷ΣaΘΪbΘΫ12ΓΘœρΗΟ»ή“Κ÷–÷πΒΈΦ”»κpHΘΫcΒΡ―ΈΥαΘ§≤βΒΟΜλΚœ»ή“ΚΒΡ≤ΩΖ÷pH»γœ¬±μΥυ ΨΘΚ
–ρΚ≈ | «β―θΜ·±Β»ή“Κ ΒΡΧεΜΐ/mL | ―ΈΥαΒΡΧεΜΐ/mL | »ή“ΚΒΡpH |
ΔΌ | 22.00 | 0.00 | 8 |
ΔΎ | 22.00 | 18.00 | 7 |
Δέ | 22.00 | 22.00 | 6 |
ΦΌ…η»ή“ΚΜλΚœ«ΑΚσΒΡΧεΜΐ±δΜ·Κω¬‘≤ΜΦΤΘ§‘ρc=___________ΓΘ





