��Ŀ����
����Ŀ����ҵ���÷�������(����Cu2S��Al2O3��Fe2O3��SiO2��)��ȡ��ͭ���̷�(FeSO4��7H2O)������[KAl(SO4)2��12H2O],�����������£�
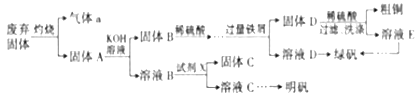
(1)����a��_________(д��ѧʽ����ͬ)���Լ�X��_________(��֪X��һ����),����C��_________��
(2)�������Ծ�ˮ��������ˮ��ԭ����____________________________________(�����ӷ���ʽ��ʾ)��
(3)���÷�Ӧ2Cu+O2+2H2SO4![]() 2CuSO4+2H2O���Ʊ�CuSO4,�����÷�Ӧ��Ƴ�ԭ��أ��õ�ص�������ӦʽΪ_______________________��
2CuSO4+2H2O���Ʊ�CuSO4,�����÷�Ӧ��Ƴ�ԭ��أ��õ�ص�������ӦʽΪ_______________________��
(4)ȡ������Ʒ�̷�,��ˮ�ܽ���ٵ��뼸��KSCN��Һ������Ʒ�̷��в�����Fe3+���۲쵽������Ϊ_________�����ڲ�Ʒ�̷���ˮ��Һ�еμ����Ը��������Һ���۲쵽������Ϊ__________________,ԭ����____________________________ (�����ӷ���ʽ��ʾ)��
(5)��֪�̷����ȷֽ�Ļ�ѧ����ʽΪ2FeSO4��7H2O![]() Fe2O3+SO2��+SO3��+14H2O����ȡ3.5g��Ʒ�̷������ȷֽ������غ�(�������Ȳ��ֽ�)������Ӧ���õ����建��ͨ��ʢ����������ˮ����ƿ�У�����0.1000 mol��L-1������KMnO4��Һ�ζ����ζ����յ�ʱ��������KMnO4��Һ�����Ϊ25.00mL,���Ʒ�̷��Ĵ���Ϊ__________ (������λ��Ч����)��
Fe2O3+SO2��+SO3��+14H2O����ȡ3.5g��Ʒ�̷������ȷֽ������غ�(�������Ȳ��ֽ�)������Ӧ���õ����建��ͨ��ʢ����������ˮ����ƿ�У�����0.1000 mol��L-1������KMnO4��Һ�ζ����ζ����յ�ʱ��������KMnO4��Һ�����Ϊ25.00mL,���Ʒ�̷��Ĵ���Ϊ__________ (������λ��Ч����)��
���𰸡� SO2 KHSO4 H2SiO3 Al3++3H2O![]() Al(OH)3(����)+3H+ O2+4e-+4H+=-2H2O ��Һ����� ���������Һ����ɫ��ȥ 5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O 99.3%
Al(OH)3(����)+3H+ O2+4e-+4H+=-2H2O ��Һ����� ���������Һ����ɫ��ȥ 5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O 99.3%
�����������ݷ�������ijɷּ����̣�����aΪSO2������B�к���CuO��Fe2O3����ҺB�к���KAlO2��K2SiO3���Լ�X��һ���Σ���ΪKHSO4������CΪH2SiO3�����������м����DΪCu��Fe����ҺDΪFeSO4��(1)����a��SO2���Լ�X��KHSO4,����C��H2SiO3��(2)�������Ծ�ˮ��������ˮ��ԭ����Al3++3H2O![]() Al(OH)3(����)+3H+��(3)���÷�Ӧ2Cu+O2+2H2SO4
Al(OH)3(����)+3H+��(3)���÷�Ӧ2Cu+O2+2H2SO4![]() 2CuSO4+2H2O��Ƴ�ԭ��أ����������õ�������������������ˮ���缫��ӦʽΪO2+4e-+4H+=-2H2O��(4)ȡ������Ʒ�̷�,��ˮ�ܽ���ٵ��뼸��KSCN��Һ����Һ����죬���Ʒ�̷��в�����Fe3+�����ڲ�Ʒ�̷���ˮ��Һ�еμ����Ը��������Һ��������Ӧ5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O���ʸ��������Һ����ɫ��ȥ��(5)���ݷ�Ӧ2FeSO4��7H2O
2CuSO4+2H2O��Ƴ�ԭ��أ����������õ�������������������ˮ���缫��ӦʽΪO2+4e-+4H+=-2H2O��(4)ȡ������Ʒ�̷�,��ˮ�ܽ���ٵ��뼸��KSCN��Һ����Һ����죬���Ʒ�̷��в�����Fe3+�����ڲ�Ʒ�̷���ˮ��Һ�еμ����Ը��������Һ��������Ӧ5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O���ʸ��������Һ����ɫ��ȥ��(5)���ݷ�Ӧ2FeSO4��7H2O![]() Fe2O3+SO2��+SO3��+14H2O��5SO2+2MnO4-+2H2O=5SO42-+2Mn2++4H+����ϵΪ10FeSO4��7H2O ~~5SO2-~~2MnO4-����Ʒ�̷��Ĵ���Ϊ
Fe2O3+SO2��+SO3��+14H2O��5SO2+2MnO4-+2H2O=5SO42-+2Mn2++4H+����ϵΪ10FeSO4��7H2O ~~5SO2-~~2MnO4-����Ʒ�̷��Ĵ���Ϊ![]() ��
��

 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д�����Ŀ�������(LiCoO2)�����һ��Ӧ�ù㷺�����͵�Դ������к�����������������̼�ȵ��ʡ�ʵ���ҳ��ԶԷϾ�����﮵�ػ��������á�ʵ�����������

��֪������ԭ����Cl->Co2+��
��Fe3+��C2O42-������ɽ��ȶ���[ Fe(C2O4)3]3-����ǿ���������·ֽ���������Fe3+���ش�����������
��1���Ͼɵ�س�������Ϊ��ĩ״��Ŀ����________________________________________��
��2���Ӻ�����Һ�õ�Al(OH)3�����ӷ�Ӧ����ʽΪ__________________________________��
��3����ҺA�е����ʳ�HCl��LiCl���__________���ѧʽ����д��LiCoO2�����ᷴӦ�Ļ�ѧ����ʽ__________________________________��
��4����������Ҫ�ɷ�Ϊ____________________���ѧʽ����
��5���ڿ����м���һ��������CoC2O4��2H2O������Ʒʱ�������ʧ�������ݼ��±����벹�������������⡣
��֪����CoC2O4�ڿ����м���ʱ���������ΪCO2
������ʧ����=��Ӧ�¶�����Ʒʧ�ص�����/��Ʒ�ij�ʼ����
��� | �¶ȷ�Χ/�� | ��ѧ����ʽ | ����ʧ���� |
�� | 120-220 | CoC2O4��2H2O | 19.67% |
�� | 300~350 | _________________________ | 59.02% |
��6����֪Li2CO3���ܶȻ�����Ksp=8.64��10-4����Ũ��Ϊ0.02mol��L-1��Li2SO4��Ũ��Ϊ0.02 mol��L-1��Na2CO3��Һ��������������Һ�е�Li+Ũ��Ϊ___________mol��L-1
��7����FeCl3��Һ�õ�FeCl3��6H2O����IJ����ؼ���_________________________��