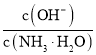��Ŀ����
����Ŀ������������(H2N2O2)��һ�ֶ�Ԫ�ᣬ��������N2O���塣
��1�������������е�Ԫ�صĻ��ϼ�Ϊ_____________________��
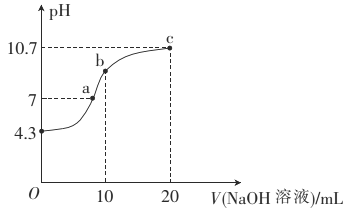
��2�������£���0��01mol��L-1��NaOH��Һ�ζ�10mL0��01mol��L-1��H2N2O2��Һ�������ҺpH��NaOH��Һ����Ĺ�ϵ��ͼ��ʾ��
��д��H2N2O2��ˮ��Һ�еĵ��뷽��ʽ��______________��
��b��ʱ��Һ��c(H2N2O2)_____�������������������� ����������ͬ��c(N2O22-)��
��a��ʱ��Һ��c(Na+)____c(HN2O2-)+c(N2O22-)��
��3����������Һ����������������Һ��ϣ����Եõ���ɫ����������������������÷�ɢϵ�еμ���������Һ������ɫ�����ͻ�ɫ��������ʱ����ɢϵ��![]() =______��[��֪Ksp(Ag2N2O2)=4��2��10-9��Ksp(Ag2SO4)=1��4��10-5]
=______��[��֪Ksp(Ag2N2O2)=4��2��10-9��Ksp(Ag2SO4)=1��4��10-5]
���𰸡�+1 H2N2O2![]() H++HN2O2- > > 3.0��10-4
H++HN2O2- > > 3.0��10-4
��������
��1�����κλ�����������Ԫ�ص��������ϼ۵Ĵ����͵���0��������������(H2N2O2)��HԪ�ػ��ϼ���+1�ۣ�OԪ�ػ��ϼ���-2�ۣ�����NԪ�صĻ��ϼ���+1�ۣ�
��2�� 0��01mol��L-1��H2N2O2��Һ��c(H2N2O2)= 0��01mol/L������Һ��pH=4��3����c(H+)=10-4��3mol/L< 0��01mol/L������H2N2O2�Ƕ�Ԫ���ᡣ�ٶ�Ԫ����ĵ���ֲ����У�����H2N2O2��ˮ��Һ�еĵ��뷽��ʽ�ǣ�H2N2O2![]() H++HN2O2-����������������NaOH��������кͷ�Ӧ�����ڶ��ߵ����ʵ���Ũ����ȣ�������NaOH��Һ�������10mLʱ��ǡ�÷�����Ӧ��H2N2O2+NaOH= NaHN2O2+ H2O��NaHN2O2��ǿ�������Σ�����Һ��HN2O2-���ڵ������ã�HN2O2-
H++HN2O2-����������������NaOH��������кͷ�Ӧ�����ڶ��ߵ����ʵ���Ũ����ȣ�������NaOH��Һ�������10mLʱ��ǡ�÷�����Ӧ��H2N2O2+NaOH= NaHN2O2+ H2O��NaHN2O2��ǿ�������Σ�����Һ��HN2O2-���ڵ������ã�HN2O2-![]() H++N2O22-���������N2O22-��H+��ʹ��Һ�����ԣ�Ҳ����ˮ�����ã�HN2O2-+H2O
H++N2O22-���������N2O22-��H+��ʹ��Һ�����ԣ�Ҳ����ˮ�����ã�HN2O2-+H2O![]() H2N2O2+OH-��ˮ�����H2N2O2��OH-��ʹ��Һ�Լ��ԣ�����ͼʾ��֪��Һ��pH>7����Һ�Լ��ԣ�˵��ˮ�����ô�����������ã�������Һ���������ʵ���Ũ�ȹ�ϵ�ǣ�c(H2N2O2)> c(N2O22-)������a�㣬�ڸ���Һ��ͬʱ���ڵ���غ㣺c(Na+)+ c(H+)=c(OH-)+c(HN2O2-)+2c(N2O22-)�����ڴ�ʱ��Һ��pH=7����Һ�����ԣ�c(H+)=c(OH-)�����ԣ�c(Na+))= c(HN2O2-)+2c(N2O22-)�����c(Na+))> c(HN2O2-)+c(N2O22-)��
H2N2O2+OH-��ˮ�����H2N2O2��OH-��ʹ��Һ�Լ��ԣ�����ͼʾ��֪��Һ��pH>7����Һ�Լ��ԣ�˵��ˮ�����ô�����������ã�������Һ���������ʵ���Ũ�ȹ�ϵ�ǣ�c(H2N2O2)> c(N2O22-)������a�㣬�ڸ���Һ��ͬʱ���ڵ���غ㣺c(Na+)+ c(H+)=c(OH-)+c(HN2O2-)+2c(N2O22-)�����ڴ�ʱ��Һ��pH=7����Һ�����ԣ�c(H+)=c(OH-)�����ԣ�c(Na+))= c(HN2O2-)+2c(N2O22-)�����c(Na+))> c(HN2O2-)+c(N2O22-)��
��3��2Ag++ N2O22-=Ag2 N2O2����Ksp(Ag2N2O2)=c2(Ag+)��c(N2O22-)=4��2��10-9������÷�ɢϵ�еμ���������Һ������������Ӧ��2Ag++ SO42-=Ag2SO4����Ksp(Ag2SO4)= c2(Ag+)��c(SO42-)=1��4��10-5������ɫ�����ͻ�ɫ��������ʱ��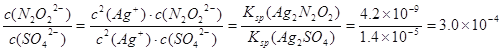 ��
��