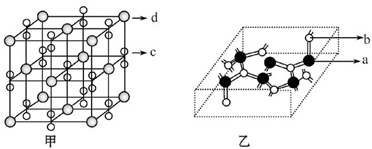
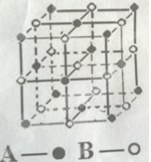
��Ŀ����
A��B��C��D���ֶ�����Ԫ�أ�ԭ��������������Aԭ�ӵ����������4�����ӣ�B�������Ӻ�C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ����Ԫ�صĵ��ʷ�Ӧ��������һ�ֵ���ɫ�Ĺ���E��D��L�����������K��M�������Ӳ��ϵĵ�����֮�͡�
��1��AΪ ��BΪ ��CΪ ��DΪ ������Ԫ�ط��ţ�
��2��D������������ˮ����Ļ�ѧʽ�� ��E�ĵ���ʽ�� ��
��3��д����A��B��ɵĻ�������E��Ӧ�Ļ�ѧ����ʽ�� ��
��4�����õ���ʽ��ʾ��C��D�γɵĻ�����F���γɹ��̣� ��
��1��C ��O ��Na ��S
��2��H2SO4 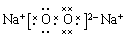
��3��2CO2 + 2Na2O2 = 2Na2CO3 + O2
��4��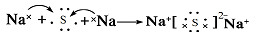
���������������1��Aԭ�ӵ����������4�����ӣ���A��������Ų�Ϊ2��4������A��CԪ�أ�B�������Ӻ�C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ����Ԫ�صĵ��ʷ�Ӧ��������һ�ֵ���ɫ�Ĺ���E����B��OԪ�أ�C��NaԪ�أ��γɵĵ���ɫ�Ĺ���E��Na2O2��D��L�����������K��M�������Ӳ��ϵĵ�����֮�͡���D��SԪ�ء���2��S�������6�����ӣ������������������ˮ����Ļ�ѧʽ��H2SO4��Na2O2�Ǻ��зǼ��Թ��ۼ������ӻ���������ʽΪ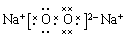 ����3����C��O��ɵĻ�����CO2��Na2O2��Ӧ�Ļ�ѧ����ʽ��2CO2 + 2Na2O2 = 2Na2CO3 + O2����4��Na��S�γɵĻ�����Na2S���γɹ��̵ĵ���ʽ��ʾΪ��
����3����C��O��ɵĻ�����CO2��Na2O2��Ӧ�Ļ�ѧ����ʽ��2CO2 + 2Na2O2 = 2Na2CO3 + O2����4��Na��S�γɵĻ�����Na2S���γɹ��̵ĵ���ʽ��ʾΪ��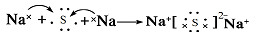 ��
��
���㣺����Ԫ�ؼ���������ƶϡ����ʵ����ʡ��ṹ���õ���ʽ��ʾ�����P�γɵĹ��̵�֪ʶ��

��14�֣��±�ΪԪ�����ڱ���һ���֣�
 �� ������ | | | | |||||
| 1 | �� | | | | | | | |
| 2 | | | | | | �� | | |
| 3 | �� | | | �� | | �� | �� | |
��1��д��Ԫ�آ������ڱ��е�λ�ã��������� ������
��2���ڢۢݵ�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ�������� ������
��3���ܢݢ���̬�⻯����ȶ�����ǿ������˳���������� ����
��4���٢ڢۢ��е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д���������ֻ�
����ĵ���ʽ�������� ��������
������������Ԫ����ɵ����ʼ䣬��һ�������£����Է�����ͼ�еı仯������A��һ
�ֵ���ɫ���塣��
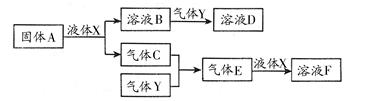
��1��д������A��Һ��X��Ӧ�����ӷ���ʽ�������������� ����������
��2������Y��һ�ִ�����Ⱦ�ֱ���ŷŻ��γ����ꡣ������ҺB���գ���B��Y���ʵ���֮��Ϊ1��1��ǡ����ȫ��Ӧʱ��������ҺD������Ϊ���� �������ѧʽ������֪��ҺD�����ԣ���D��Һ�и�������Ũ���ɴ�С��˳��Ϊ ����
��3����100 mL 18 mol/L��FŨ��Һ�м������ͭƬ������ʹ֮��ַ�Ӧ��������������������£�����Ϊ��������������������
A��40.32 L B��30.24 L C��20.16 L D��13.44 L
�±�ΪԪ�����ڱ���һ���֣�
| �� ���� | | | | |||||
| 1 | �� | | | | | | | |
| 2 | | | | | | �� | | |
| 3 | �� | | | �� | | �� | �� | |
�������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺
��1��д��Ԫ�آڵ����ӽṹʾ��ͼ______________��
��2���ڡ��ۡ��ݵ����Ӱ뾶�ɴ�С��˳��Ϊ_________________________��
��3��Ԫ�آ�����γɻ�����ĵ���ʽ��_________________________��
������������Ԫ����ɵ����ʼ䣬��һ�������£����Է�����ͼ��ʾ�ı仯������A��һ�ֵ���ɫ���塣��ش�
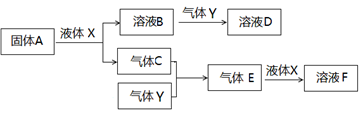
��4��д������A��Һ��X��Ӧ�����ӷ���ʽ ��
��5������Y��һ�ִ�����Ⱦ�ֱ���ŷŻ��γ����ꡣ������ҺB���գ���B��Y���ʵ���֮��Ϊ1:1��ǡ����ȫ��Ӧʱ��������ҺD����֪��ҺD�����ԣ���D��Һ�и�������Ũ���ɴ�С��˳��Ϊ ��
��6����500�棬101kPaʱ������C������Y��Ӧ����0��2mol����Eʱ���ų�akJ������д���������·�Ӧ���Ȼ�ѧ����ʽ ��
��7��������C��Y�ں��ݾ��ȵ������·�Ӧ������˵�����жϴﵽƽ��״̬���� ��
A���¶Ȳ��� B��������ѹǿ���� C�����������ܶȲ��� D����������ƽ������������
�±�Ϊ���ֶ�����Ԫ�ػ��ϼۼ���Ӧԭ�Ӱ뾶�����ݣ�
| Ԫ������ | Ԫ�ر�� | |||||||
| A | B | C | D | E | F | G | H | |
| ԭ�Ӱ뾶(nm) | 0.102 | 0.110 | 0.117 | 0.074 | 0.075 | 0.071 | 0.099 | 0.077 |
| ����ϼ� | ��6 | ��5 | ��4 | | ��5 | | ��7 | ��4 |
| ��ͻ��ϼ� | ��2 | ��3 | ��4 | ��2 | ��3 | ��1 | ��1 | ��4 |
��֪��
��A��D���γɻ�����AD2��AD3��
��E��D���γɶ��ֻ��������ED��ED2�dz����Ļ����C�������ƹ��ء�
(1)E�����ڱ���λ���� ��
(2)C��H����̬�⻯����ȶ���ǿ����ϵΪ (�÷���ʽ��ʾ)��
(3)�������ΪADG2��������ˮ�л�ǿ��ˮ�⣬����ʹƷ����Һ��ɫ����ɫ�����һ��ǿ�ᡣ�÷�Ӧ�Ļ�ѧ����ʽ�� ��
(4)��ҵ�Ͽ��ô�����Һ����ED��ED2���÷�Ӧ���£�
ED��ED2��Na2CO3=2 ��CO2
������ij�εĻ�ѧʽӦΪ ��
(5)��һ�ܱ������з�����Ӧ2AD2��D2
 2AD3 ��H����47 kJ/mol��������ƽ����ϵ�м���18D2����ƽ�ⷢ���ƶ���AD2��18D�İٷֺ��� (����ӡ������١����䡱)��ԭ��Ϊ ��
2AD3 ��H����47 kJ/mol��������ƽ����ϵ�м���18D2����ƽ�ⷢ���ƶ���AD2��18D�İٷֺ��� (����ӡ������١����䡱)��ԭ��Ϊ ��(6)�����һ��ʵ�鷽����ʹͭ��ϡ��H2AD4��Һ��Ӧ���õ���ɫ��Һ�������������ʵ�鷽��װ��ͼ��