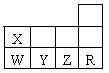
��Ŀ����
���ֶ����ڵ�����Ԫ��A��B��C��D������ԭ�ӵĵ��Ӳ���������������֮�ȷֱ���3 :1��l : l��1 : 2��l :3��
�ݴ˻ش��������⣺
(1) д��A��D��Ԫ�ط��ţ�A ��D ��
(2)����������������Ϣ�Ķ�����Ԫ���У�����������ˮ����������ǿ����(�ѧʽ) ����A��D��Ԫ�ع��ɵĻ�����A2D2�ĺ��еĻ�ѧ������ ��
(3)д����������������Ϣ�Ļ�����CD2�뻯����A2D2��Ӧ�Ļ�ѧ����ʽ��
(4)��B��C��A����ͬһ���ڣ���B��C�Ļ�������ˮ��Ӧ�Ļ�ѧ����ʽΪ��
�ݴ˻ش��������⣺
(1) д��A��D��Ԫ�ط��ţ�A ��D ��
(2)����������������Ϣ�Ķ�����Ԫ���У�����������ˮ����������ǿ����(�ѧʽ) ����A��D��Ԫ�ع��ɵĻ�����A2D2�ĺ��еĻ�ѧ������ ��
(3)д����������������Ϣ�Ļ�����CD2�뻯����A2D2��Ӧ�Ļ�ѧ����ʽ��
(4)��B��C��A����ͬһ���ڣ���B��C�Ļ�������ˮ��Ӧ�Ļ�ѧ����ʽΪ��
(14��)(1)A Na ��2�֣���D O ��2�֣���
(2)H2SO4 ��2�֣������Ӽ����ۼ��������Ӽ��ͷǼ��Լ�����2�֣�
(3)2CO2+2Na2O2=2Na2CO3+O2 ��2�֣� SO2+ Na2O2=Na2SO4��2�֣�
(4)Al2S3+6H2O=2Al(OH)3��+3H2S����2�֣�
(2)H2SO4 ��2�֣������Ӽ����ۼ��������Ӽ��ͷǼ��Լ�����2�֣�
(3)2CO2+2Na2O2=2Na2CO3+O2 ��2�֣� SO2+ Na2O2=Na2SO4��2�֣�
(4)Al2S3+6H2O=2Al(OH)3��+3H2S����2�֣�
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
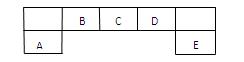
 ��1��B��C��DԪ�ص縺�ԵĴ�С˳��Ϊ��______��______��______(��Ԫ�ط���)��
��1��B��C��DԪ�ص縺�ԵĴ�С˳��Ϊ��______��______��______(��Ԫ�ط���)��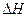 = (ע���������赥�ʾ�Ϊ���ȶ�����)
= (ע���������赥�ʾ�Ϊ���ȶ�����)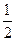 O2(g)��XO2(g)+283.0 kJ��mol
O2(g)��XO2(g)+283.0 kJ��mol ʽ��1s22s22p63s23p63d104s24p1��
ʽ��1s22s22p63s23p63d104s24p1�� ����Ϊ ��
����Ϊ ��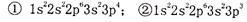
 ���бȽ��У���ȷ����
���бȽ��У���ȷ����