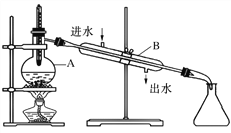
��Ŀ����
����Ŀ��ʵ��������500mL0.1mol/LNa2CO3��Һ���ش���������
��1������Na2CO3��Һʱ���õ���Ҫ������������ƽ����ֽ���ձ���ҩ�ס�___��
��2������ƿ�ϱ��п̶��ߡ�___��ʹ��ǰҪ___��
��3������������ƽ��ȡNa2CO3____g��
��4����ʵ���������������Һ��Ũ����ƫ�ߣ�ƫ�ͻ��Dz��䣿
A.��ˮʱ�����̶���___��
B.�ܽ��δ��ȴ�����¾�ת������ƿ___��
C.����ƿ�ڱڸ���ˮ���δ���ﴦ��___��
D.����ʱ����___��
E.���µߵ�ҡ�Ⱥ�Һ����ڿ���___��
��5����ʵ������Ҫ��Ũ��Ϊ16mol/L��Ũ��������480mL2.0mol/L��ϡ���ᣬ����Ҫ��ȡŨ��������Ϊ___mL��
���𰸡�500mL����ƿ������������ͷ�ι� �¶ȡ��ݻ� ��© 5.3 ƫ�� ƫ�� ���� ƫ�� ���� 62.5
��������
����һ�����ʵ���Ũ����Һ�����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���������ʱ�ɸ���c=![]() �жϡ�
�жϡ�
(1)�������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ�����ҡ�ȡ����ݡ�ҡ�ȡ�װƿ��֪����������У�������ƽ���ձ�����������ҩ�ס�500mL����ƿ�ͽ�ͷ�ιܣ�ȱ�ٵ�������500mL����ƿ����ͷ�ιܡ���������
(2)����ƿ�ϱ����¶ȡ��̶��ߡ��ݻ�������ƿ����ƿ����Ϊ��ֹʹ�ù�����©Һ��ʹ��ǰӦ��©��
(3)����500mL0.1mol/LNa2CO3����ҪNa2CO3������Ϊ��0.5L��0.1mol/L��106g/mol=5.3g��
(4)A����ˮʱ�����̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ�
B���ܽ��δ��ȴ�����¾�ת������ƿ����ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ�
C������ƿ�ڱڸ���ˮ���δ���ﴦ�������������ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣻
D������ʱ���ӣ�������Һ���ƫ����ҺŨ��ƫ�ͣ�
E�����µߵ�ҡ�Ⱥ�Һ����ڿ��ߣ�����������������ҺŨ�Ȳ��䣻
(5)��ʵ������Ҫ��Ũ��Ϊ16mol/L��Ũ��������480mL2.0mol/L��ϡ���ᣬӦѡ��500mL����ƿ������ҪŨ�������ΪV����������Һϡ�������������ʵ�������ã�16mol/L��V=500mL2.0mol/L�����V=62.5mL��

����Ŀ������������������Һ���й���������ȷ����
��� | �� | �� | �� | �� |
pH | 11 | 11 | 3 | 3 |
��Һ | ��ˮ | ����������Һ | ���� | ���� |
A. �ۢ��зֱ���������Ĵ����ƾ��������Һ��pH������
B. �ڢ�����Һ�������ϣ�������Һ��c��H+��>c��OH-��
C. �ֱ��ˮϡ��10����������Һ��pH����>��>��>��
D. �������Һ�У�ˮ�ĵ���̶ȣ���>��