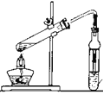
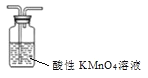
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ“ΜΕ®Έ¬Ε»ΖΕΈßΡΎ”Ο¬»Μ·ΡΤ»έΫΰΦΊ≥Λ ·Θ®÷ς“Σ≥…ΖίΈΣKAlSi3O8Θ©Ω…÷ΤΒΟ¬»Μ·ΦΊΘ§÷ς“ΣΖ¥”Π «ΘΚNaCl(l)+KAlSi3O8(s) ![]() KCl(l)+NaAlSi3O8(s)Θ§Άξ≥…œ¬Ν–ΧνΩ’ΘΚ
KCl(l)+NaAlSi3O8(s)Θ§Άξ≥…œ¬Ν–ΧνΩ’ΘΚ
Θ®1Θ©…œ ωΖ¥”Π…φΦΑΒΡΒΎ»ΐ÷ήΤΎ‘ΣΥΊ÷–Θ§άκΉ”ΑκΨΕΉν–ΓΒΡ «___ΘΜCl‘≠Ή””κSi‘≠Ή”Ω…ΙΙ≥…”–5Ηω‘≠Ή”ΚΥΒΡΖ÷Ή”Θ§ΤδΖ÷Ή”ΒΡΩ’ΦδΙΙ–ΆΈΣ____ΓΘ
Θ®2Θ©”ΟΉνœξΨΓΟη ωΚΥΆβΒγΉ”‘ΥΕ·Ή¥Χ§ΒΡΖΫ ΫΘ§±μ Ψ―θάκΉ”ΚΥΆβΒγΉ”ΒΡ‘ΥΕ·Ή¥Χ§_____ΓΘ
Θ®3Θ©NaΚΆO2Ζ¥”Π–Έ≥…Na2OΚΆNa2O2ΒΡΜλΚœΈοΘ§“θ―τάκΉ”ΒΡΗω ΐ±»ΈΣ__ΘΜNaAlSi3O8ΗΡ–¥≥…―θΜ·Έο–Έ Ϋ «___ΓΘ
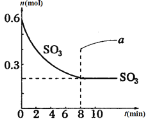
Θ®4Θ©Ρ≥–Υ»Λ–ΓΉιΈΣ―–ΨΩ…œ ωΖ¥”Π÷–ΦΊ‘ΣΥΊΒΡ»έ≥ω¬ Θ®“ΚΧε÷–ΦΊ‘ΣΥΊΒΡ÷ ΝΩ’Φ―υΤΖ÷ ΝΩΖ÷ ΐΘ©”κΈ¬Ε»ΒΡΙΊœΒΘ§Ϋχ–– Β―ιΘ®±Θ≥÷ΤδΥϋΧθΦΰ≤Μ±δΘ©Θ§ΜώΒΟ»γœ¬ ΐΨίΘΚ
1.5 | 2.5 | 3.0 | 3.5 | 4.0 | |
800Γφ | 0.054 | 0.091 | 0.127 | 0.149 | 0.165 |
830Γφ | 0.481 | 0.575 | 0.626 | 0.669 | 0.685 |
860Γφ | 0.515 | 0.624 | 0.671 | 0.690 | 0.689 |
950Γφ | 0.669 | 0.711 | 0.713 | 0.714 | 0.714 |
Ζ÷Έω ΐΨίΩ…“‘ΒΟ≥ωΘ§¬»Μ·ΡΤ»έΫΰΦΊ≥Λ · «__________Ζ¥”ΠΘ®ΧνΓΑΖ≈»»Γ±ΜρΓΑΈϋ»»Γ±Θ©ΘΜ‘Ύ950Γφ ±Θ§”ϊΧαΗΏ»έ≥ωΦΊΒΡΥΌ¬ Ω…“‘≤…»ΓΒΡ“Μ÷÷¥κ © «_______ΓΘ
Θ®5Θ©Na(l)ΘΪKCl(l) ![]() NaCl(l)ΘΪK(g) «ΙΛ“Β…œ“±ΝΕΫπ τΦΊ≥Θ”ΟΒΡΖΫΖ®Θ§ΗΟΖΫΖ®Ω…––ΒΡ‘≠“ρ «___ΓΘ
NaCl(l)ΘΪK(g) «ΙΛ“Β…œ“±ΝΕΫπ τΦΊ≥Θ”ΟΒΡΖΫΖ®Θ§ΗΟΖΫΖ®Ω…––ΒΡ‘≠“ρ «___ΓΘ
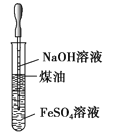
Θ®6Θ©¬ΝΩ…”Ο”Ύ“±ΝΕΡ―»έΫπ τΘ§άϊ”Ο¬ΝΒΡ«Ή―θ–‘Θ§ΜΙΩ…”Ο”Ύ÷Τ»ΓΡΆΗΏΈ¬ΒΡΫπ τΧ’¥…ΓΘάΐ»γΫΪ¬ΝΖέΓΔ ·ΡΪΚΆΕΰ―θΜ·ν―Α¥“ΜΕ®±»άΐΜλΚœΨυ‘»Θ§ΆΩ‘ΎΫπ τ±μΟφ…œΘ§»ΜΚσ‘ΎΗΏΈ¬œ¬λ―…’Θ§Ω…‘ΎΫπ τ±μΟφ–Έ≥…ΡΆΗΏΈ¬ΒΡΆΩ≤ψTiCΘ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ_____ΓΘ
ΓΨ¥πΑΗΓΩAl3+ ’ΐΥΡΟφΧε ![]() 1©U2 Na2OΓΛAl2O3ΓΛ6SiO2 Έϋ»» ≥δΖ÷ΫΝΑηΜρΫΪΦΊ≥Λ ·ΖέΥι≥…Ηϋ–ΓΒΡΩ≈ΝΘ ΗυΨίά’œΡΧΊΝ–‘≠άμΘ§ΫΪΦΊ’τΤχΖ÷άκ≥ωά¥Θ®ΫΒΒΆΝΥ≤ζΈοΒΡ≈®Ε»Θ©Θ§Μ·―ßΤΫΚβœρ’ΐΖ¥”ΠΖΫœρ“ΤΕ· 4Al+3TiO2+3C
1©U2 Na2OΓΛAl2O3ΓΛ6SiO2 Έϋ»» ≥δΖ÷ΫΝΑηΜρΫΪΦΊ≥Λ ·ΖέΥι≥…Ηϋ–ΓΒΡΩ≈ΝΘ ΗυΨίά’œΡΧΊΝ–‘≠άμΘ§ΫΪΦΊ’τΤχΖ÷άκ≥ωά¥Θ®ΫΒΒΆΝΥ≤ζΈοΒΡ≈®Ε»Θ©Θ§Μ·―ßΤΫΚβœρ’ΐΖ¥”ΠΖΫœρ“ΤΕ· 4Al+3TiO2+3C![]() 2Al2O3+3TiC
2Al2O3+3TiC
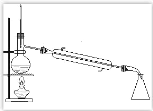
ΓΨΫβΈωΓΩ
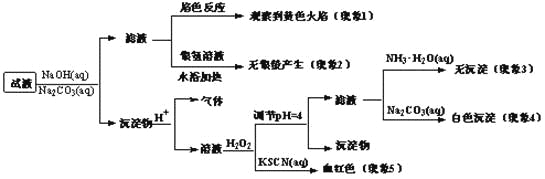
Θ®1Θ©ΒγΉ”≤ψΫαΙΙœύΆ§ΒΡάκΉ”Θ§ΚΥΒγΚ… ΐ‘Ϋ¥σάκΉ”ΑκΨΕ‘Ϋ–ΓΘ§άκΉ”ΒγΉ”≤ψ‘ΫΕύάκΉ”ΑκΨΕ‘Ϋ¥σΘ§Ι Cl->Na+>Al3+ΘΜCl‘≠Ή””κSi‘≠Ή”Ω…ΙΙ≥…”–5Ηω‘≠Ή”ΚΥΒΡΖ÷Ή”ΈΣSiCl4Θ§Si–Έ≥…4ΗωSi-ClΦϋΘ§ΟΜ”–Ι¬Ε‘ΒγΉ”Θ§ΙηΈΣ’ΐΥΡΟφΧεΙΙ–ΆΘ§Ι ¥πΑΗΈΣΘΚAl3+ΘΜ’ΐΥΡΟφΧεΘΜ
Θ®2Θ©ΉνœξΨΓΟη ωΚΥΆβΒγΉ”‘ΥΕ·Ή¥Χ§ΒΡΖΫ ΫΈΣΙλΒά±μ Ψ ΫΘ§±μ Ψ―θάκΉ”ΚΥΆβΒγΉ”ΒΡ‘ΥΕ·Ή¥Χ§ΘΚ![]() Θ§Ι ¥πΑΗΈΣΘΚ
Θ§Ι ¥πΑΗΈΣΘΚ![]() ΘΜ
ΘΜ
(3)Na2OΚΆNa2O2ΒΡ“θ―τάκΉ” ΐΡΩ÷°±»ΨυΈΣ1ΘΚ2Θ§Ι Εΰ’ΏΜλΚœΈο÷–“θ―τάκΉ” ΐΡΩ÷°±»ΈΣ1ΘΚ2ΘΜNaAlSi3O8ΗΡ–¥≥…―θΜ·Έο–Έ Ϋ «Na2OAl2O36SiO2Θ§
Ι ¥πΑΗΈΣΘΚ1ΘΚ2ΘΜNa2OΓΛAl2O3ΓΛ6SiO2ΘΜ
Θ®4Θ©”…±μ÷– ΐΨίΩ…÷ΣΘ§Έ¬Ε»‘ΫΗΏΦΊ‘ΣΥΊΒΡ»έ≥ω¬ ‘ΫΗΏΘ§ΥΒΟς…ΐΗΏΈ¬Ε»Θ§ΤΫΚβœρ’ΐΖ¥”ΠΖΫœρ“ΤΕ·Θ§…ΐΗΏΈ¬Ε»ΤΫΚβœρΈϋ»»ΖΫœρ“ΤΕ·Θ§Ι ’ΐΖ¥”ΠΈΣΈϋ»»Ζ¥”ΠΘ§ΗΟΉΣΜ·Ιΐ≥ΧΟΜ”–ΤχΧε≤Έ”κΘ§ ΙΖ¥”ΠΈο≥δΖ÷Ϋ”¥ΞΩ…“‘ΧαΗΏΖ¥”ΠΥΌ¬ Θ§Ω…“‘≥δΖ÷ΫΝΑηΘ§ΫΪΦΊ≥Λ ·ΖέΥι≥…Ηϋ–ΓΒΡΩ≈ΝΘΘ§Ι ¥πΑΗΈΣΘΚΈϋ»»ΘΜ≥δΖ÷ΫΝΑηΘ§ΫΪΦΊ≥Λ ·ΖέΥι≥…Ηϋ–ΓΒΡΩ≈ΝΘΘΜ
Θ®5Θ©ΗυΨίά’œΡΧΊΝ–‘≠άμΘ§ΫΪΦΊ’τΤχΖ÷άκ≥ωά¥(ΫΒΒΆΝΥ≤ζΈοΒΡ≈®Ε»)Θ§Μ·―ßΤΫΚβœρ’ΐΖ¥”ΠΖΫœρ“ΤΕ·Θ§
Ι ¥πΑΗΈΣΘΚΗυΨίά’œΡΧΊΝ–‘≠άμΘ§ΫΪΦΊ’τΤχΖ÷άκ≥ωά¥(ΫΒΒΆΝΥ≤ζΈοΒΡ≈®Ε»)Θ§Μ·―ßΤΫΚβœρ’ΐΖ¥”ΠΖΫœρ“ΤΕ·ΘΜ
Θ®6Θ©¬ΝΖέΓΔ ·ΡΪΚΆΕΰ―θΜ·ν―Α¥“ΜΕ®±»άΐΜλΚœΨυ‘»Θ§‘ΎΗΏΈ¬œ¬λ―…’–Έ≥…ΡΆΗΏΈ¬ΒΡΆΩ≤ψTiCΘ§”…‘ΣΥΊ ΊΚψ÷ΣΜΙ…ζ≥…―θΜ·¬ΝΘ§Ζ¥”ΠΖΫ≥Χ ΫΈΣΘΚ4Al+3TiO2+3C![]() 2Al2O3+3TiCΓΘ
2Al2O3+3TiCΓΘ