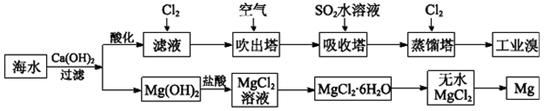
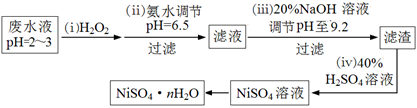
��Ŀ����
ǿ���������Ǹ��ֽⷴӦ��һ����Ҫ���ɡ�����ġ�ǿ�ᡱ�������ᡱָ���ǿ�����ܳ������Ե�һЩ���������ʣ�����ࡢ�������������ʽ�εȲ���ķ�ӦҲ�ɸ���������ǿ�����������������ж�����
(1)HA��H2B���������ᣬ�����¹�ϵ��H2B(����)��2A��=B2����2HA����A����HB����B2�����������У����������(H��)����________��
(2)����ǿ���������ʵı����й��⣬�����ܼ��йأ���CH3COOH��HF��Һ������NH3Ӱ��ɷ�����ȫ���롣��Һ����CH3COONa��HCl�D��NaCl��CH3COOH��һ��Ӧ�ܷ���________(��ܡ���)��������____________________��
(3)ijͬѧʵ�鷢�֣���H2S����ͨ��CuSO4��Һ�У����ɺ�ɫ������Ū�������CuS��д���˻�ѧ����ʽ��H2S��CuSO4=CuS����H2SO4��������������������ⲻ�������Ƶ�ǿ��������ǿ��������Ĺ���ì���ˡ������������__________________________________________��
(4)������ԭ��Ӧ��Ҳ�����ƹ��ɣ���ǿ�����������������������ʡ�����ǿ��ԭ������������ԭ�����ʡ����ݴ��ж����з�Ӧ�ܹ���������________(����ĸ���)��
A��FeCl2��Cl2 FeCl3
FeCl3
B��Fe��I2 FeI3
FeI3
C��Fe��CuSO4 FeSO4��Cu
FeSO4��Cu
D��FeCl3��Cu CuCl2��FeCl2
CuCl2��FeCl2
E��FeBr3��Cl2 FeCl2��Br2
FeCl2��Br2
F��FeI2��Br2 FeBr3��I2
FeBr3��I2
(1)A��
(2)����Ϊ��Һ���д��������ᶼ��ǿ��
(3)��ì�ܡ�ǿ��������������������ʣ����˷�Ӧ�г������ɣ�Ҳ���ϸ��ֽⷴӦ����������
(4)ACDF
����

 Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д����������Ǵ�����Ⱦ��֮һ��������������ķ����ж��֡�
��1�����ü������ԭ���������֪��
��CH4 (g)��4NO2(g)��4NO(g)��CO2(g)��2H2O(g�� ��H ����574 kJ/mol
��CH4(g)��4NO(g���� 2N2(g)��CO2(g)��2H2O(g�� ��H ����1160 kJ/mol
��CH4 ��NO2 ��ԭΪN2 ���Ȼ�ѧ����ʽΪ�� ��
��2������NH3����ԭ�������SCR����)���ü�����ĿǰӦ����㷺���������������ѳ������� ��Ӧ�Ļ�ѧ����ʽΪ: Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�� ��д��1�����ɣ���
Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�� ��д��1�����ɣ���
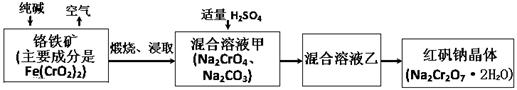
��3������ClO2�������������ת����������: NO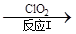 NO2
NO2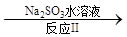 N2����֪��Ӧ��Ļ�ѧ����ʽΪ2NO+ ClO2 + H2O ��NO2 + HNO3 + HCl����Ӧ��Ļ�ѧ����ʽ�� ��������11.2 L N2����״������������ClO2 g ��
N2����֪��Ӧ��Ļ�ѧ����ʽΪ2NO+ ClO2 + H2O ��NO2 + HNO3 + HCl����Ӧ��Ļ�ѧ����ʽ�� ��������11.2 L N2����״������������ClO2 g ��
��4���û���̿��ԭ��������������йط�ӦΪ��C��s��+2NO��g�� N2 ��g��+CO2 ��g����H��ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2 ��g��+CO2 ��g����H��ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
| Ũ��/mol?L-1/ ʱ��/min | NO | N2 | CO2 |
| 0 | 0.100 | 0 | 0 |
| 10 | 0.058 | 0.021 | 0.021 |
| 20 | 0.040 | 0.030 | 0.030 |
| 30 | 0.040 | 0.030 | 0.030 |
| 40 | 0.032 | 0.034 | 0.017 |
| 50 | 0.032 | 0.034 | 0.017 |
��T1��ʱ���÷�Ӧ��ƽ�ⳣ��K= ��������λС��������30min�ı�ijһ��������Ӧ���´ﵽƽ�⣬��ı������������ ������30min�������¶���T2�棬�ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ5��3��3����÷�Ӧ�ġ�H 0�����������=����������
 2CrO42-+2H+����д����ƽ���ƽ�ⳣ������ʽK= ����������ˮϡ�ͣ�ƽ�⽫ �ƶ�(���������������)��
2CrO42-+2H+����д����ƽ���ƽ�ⳣ������ʽK= ����������ˮϡ�ͣ�ƽ�⽫ �ƶ�(���������������)��